- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Matter at extremes
- Carbon's descent into the Earth's core
Carbon's descent into the Earth's core
The presence of carbonates in the Earth's mantle is known from diamond inclusions but how carbon arrived there remains a mystery. Two new iron carbonate compounds have been synthesised and show that self-oxidation-reduction reactions could preserve carbonates in the mantle, hence becoming a potential carbon-carrier down to the Earth's core.
In the last century, the rapid increase in the amount of CO2 in the atmosphere, together with the observed climate change, have increasingly focused scientists’ attention on the carbon cycle and its evolution at the Earth’s surface. The carbon cycle also extends below the surface: recent estimations locate up to 90% of the Earth’s carbon budget in the Earth’s mantle and core [1]. Due to the dynamic nature of convection and subduction, there is a constant recycling of carbon between the Earth’s surface and its deep interior. Subduction is considered the only mechanism capable of carrying a significant amount of carbon into the deep Earth.
This study focuses on carbonate phases, which are the main carbon-bearing minerals in subducting slabs and, in particular, on the stability of Fe-carbonates such as FeCO3 (siderite). Indeed, a previous experimental investigation on MgCO3 has revealed its stability at pressures (P) and temperatures (T) down to those of the core mantle boundary (CMB) [2]. Motivated by the extensive solid solutions formed between MgCO3 and FeCO3 at ambient conditions, and the spin transition of Fe atoms at high pressures, which might influence the stability of the phase itself, high-pressure high-temperature experiments were performed on FeCO3 to study its stability and determine its decomposition or transformation products at the relative P and T conditions.
Experiments were performed using synthetic FeCO3 single crystals, at P and T conditions covering the entire Earth’s mantle, i.e. reaching above 110 GPa and 2500 K, respectively. High pressures and high temperatures were achieved by means of diamond anvil cells (DACs) and by double side laser heating. FeCO3 characterisation and of its derivatives required the use of microfocusing X-ray optics due to the small dimensions of the single crystals (10 µm2). Hence, experiments were performed at the microfocus X-ray single crystal diffraction (XRSD) beamlines ID09A (now ID15B) and ID27 as well as by the synchrotron Mössbauer source (SMS) at ID18. One experimental run was carried out at APS.
 |
|
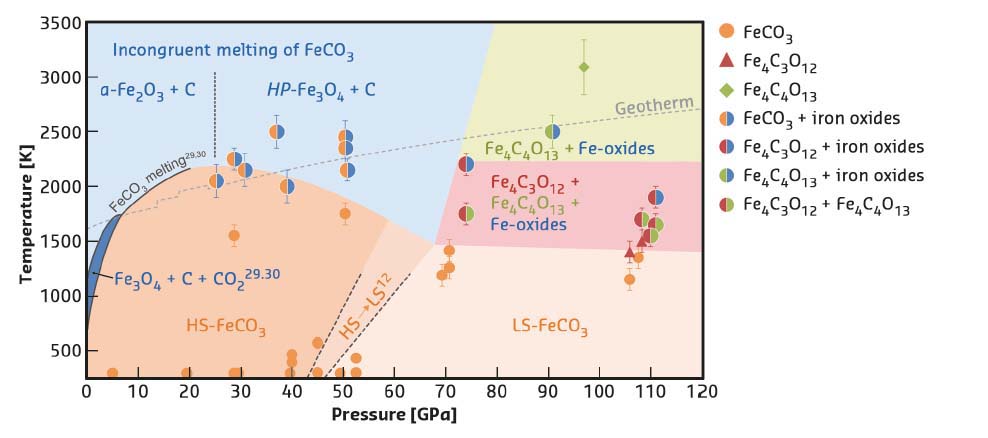
Fig. 44: Stability diagram of FeCO3 at high P-T. |
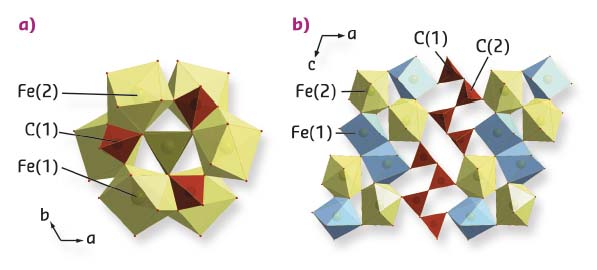
The results show that upon heating FeCO3 to Earth geotherm temperatures at pressures up to about 50 GPa, FeCO3 partially dissociates, forming different iron oxides (Figure 44). In particular, below and above ~25 GPa, dissociation leads to the formation of α-Fe2O3 (hematite) and HP-Fe3O4 (HP-magnetite). As reported by previous authors, α-Fe2O3 and HP-Fe3O4 form as a result of redox dissociation of liquid-FeCO3 leading to dissolved Fe3+ and CO2 in the carbonate melt. At pressures above ~75 GPa, two new compounds were synthesised and their structures solved – tetrairon (III) orthocarbonate; Fe43+C3O12, and diiron (II) diiron (III) tetracarbonate; Fe22+Fe23+C4O13 (Figure 45). Both materials contain CO4 tetrahedra and the first is characterised by a unique structure, thus indicating that high pressure carbonates may not be similar to any other compounds (including silicates). Based on a number of different datasets, it is inferred that the orthocarbonate, Fe4C3O12, forms directly from heating FeCO3 at T > 1400 K according to the reaction 4FeCO3 → Fe4C3O12 + C. The tetracarbonate, Fe4C4O13, appears upon prolonged heating of Fe4C3O12 above ~75 GPa but at temperatures significantly higher than those needed for the synthesis of the orthocarbonate (Figure 44) following the possible reaction 8Fe4C3O12 → 6Fe4C4O13 + 4Fe2O3 + 3O2. This reaction indicates that iron is reduced by oxygen, where something similar has already been observed in studies of iron(III) oxides at pressure above ~70 GPa.
 |
|
Fig. 45: Crystal structures of high-pressure carbonates a) Tetrairon (III) orthocarbonate Fe4C3O12 and b) diiron (II) diiron (III) tetracarbonate Fe4C4O13, at ambient temperature and 74(1) and 97(2) GPa, respectively. |
Thus, it is concluded that the tetracarbonate is the product of chemical evolution of the othocarbonate and that ferrous and ferric iron plays a role in stabilising carbonates at extreme conditions. Moreover, Fe4C4O13 was found to survive along the entire geotherm to the depth corresponding to at least 2500 km, demonstrating that self-oxidation-reduction reactions should not lead to the destruction of carbonates in the Earth’s lower mantle.
Principal publication and authors
Stability of iron-bearing carbonates in the deep Earth’s interior, V. Cerantola (a), E. Bykova (b,c), I. Kupenko (a,i) , M. Merlini (d), L. Ismailova (b,e), C. McCammon (b), M. Bykov (b,f), A. I. Chumakov (a), S. Petitgirard (b), I. Kantor (a,j), V. Svitlyk (a), J. Jacobs (a), M. Hanfland (a), M. Mezouar (a), C. Prescher (g), R. Rüffer (a), V. Prakapenka (h) and L. Dubrovinsky (b), Nat. Commun. 8, 15960 (2017); doi: 10.1038/ncomms15960.
(a) ESRF
(b) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
(c) Deutsches Elektronen-Synchrotron, Hamburg (Germany)
(d) Dipartimento di Scienze della Terra, Università degli Studi di Milano (Italy)
(e) Skolkovo Institute of Science and Technology, Skolkovo Innovation Center (Russia)
(f) Material Modeling and Development Laboratory, National University of Science and Technology MSIS, Moscow (Russia)
(g) Institute of Geology and Mineralogy, Universität zu Köln (Germany)
(h) GSECARS, Center for Advanced Radiation Sources, University of Chicago, Argonne National Laboratory (USA)
(i) Present address: Institut für Mineralogie, Universität Münster (Germany)
(j) Present address: MAX IV Laboratory, Lund (Sweden)
References
[1] B. Marty et al., Rev. Mineral. Geochem. 75, 149-181 (2013)
[2] M. Isshiki et al., Nature 427, 60-63 (2004)



