- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Matter at extremes
- Crystal structure of SiO2 polymorph cristobalite X-I: bridging the gap towards the seifertite enigma
Crystal structure of SiO2 polymorph cristobalite X-I: bridging the gap towards the seifertite enigma
Silica polymorph α-cristobalite was studied from ambient to high pressures to understand its densification mechanism and link to formation of the polymorph seifertite, only found in severely shocked meteorites. Using synchrotron radiation, the structure of an intermediate and long-disputed polymorph – cristobalite X-I – was solved, revealing the missing step in the transformation sequence.
Seiferite, α-PbO2-type silica, is the densest known SiO2 polymorph discovered so far. It is considered to be stable above ~80 GPa, which can either be accommodated at greater depths of planetary interiors or in the only natural samples available – severely shocked meteorites. In various shocked meteorites, low-pressure silica polymorph α-cristobalite is commonly observed in close spatial relation with seifertite. To understand the curious coexistence of these low- and high-pressure silica phases, the behaviour of α-cristobalite under pressure was systematically studied. The focus of the study was to constrain the conditions under which a controversial phase bridging α-cristobalite and seifertite – cristobalite X-I – can be formed. By revealing the crystal structure of the metastable polymorph cristobalite X-I, and the conditions under which it forms, it was possible to gain insight into the long-disputed transition mechanism from α-cristobalite to α-PbO2-type silica.
High-pressure experiments were carried out on both single crystals and powders. All single-crystal experiments were conducted in quasi-hydrostatic conditions, using a pressure-transmitting medium, while powders were also studied in a non-hydrostatic environment. A starting material in the form of α-cristobalite was compressed using diamond anvil cells (DAC). In situ high-pressure data were obtained by Raman spectroscopy and synchrotron-based single-crystal X-ray diffraction (SCXRD) at ESRF beamlines ID15B and ID27, and also at DESY.
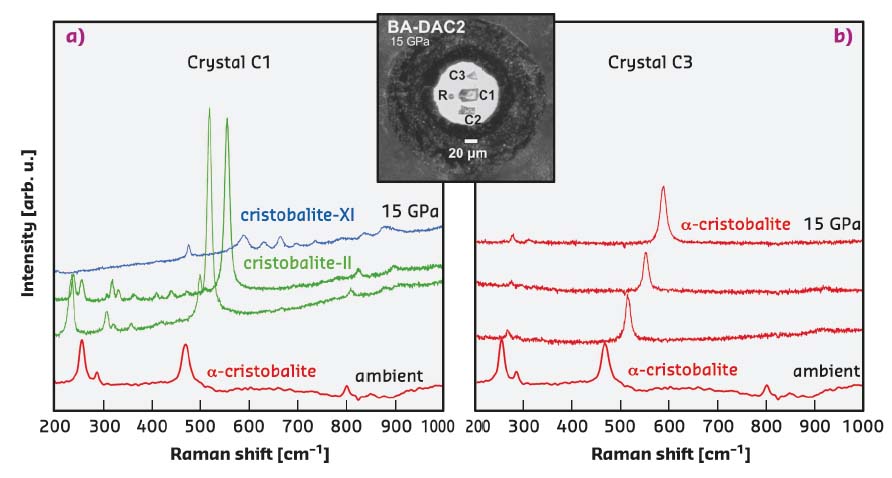
The mechanism that α-cristobalite adopts as response to compression was found to strongly depend on the stress conditions. Single crystals that remain surrounded by very soft neon upon compression, experiencing a (almost) hydrostatic environment, retain the structure of α-cristobalite up to at least ~15 GPa, as shown in Figure 46. Crystals that touch the diamond anvils upon compression, undergo a displacive phase transition to cristobalite-II near 1 GPa and further transform to cristobalite X-I just above ~10 GPa (Figure 46), in agreement with earlier findings [1]. Cristobalite X-I can be followed at least up to ~80 GPa under compression. After recovering cristobalite X-I from high-pressure experiments, it transformed back to the initial α-cristobalite. Compression experiments carried out on powders, regardless of the presence of the pressure-transmitting medium, showed identical transformation paths, ultimately resulting in the formation of seifertite-like material.
 |
|
Fig. 46: In situ Raman spectra collected on two different single crystals of α-cristobalite at 1.1, 4.6, 8.6 and 15.0 GPa and at room temperature a) in quasi-hydrostatic conditions and b) in the case of high hydrostaticity. Inset shows the DAC pressure chamber seen at 15.0 GPa: R stands for ruby; C1, C2 and C3 for different cristobalite crystals. |
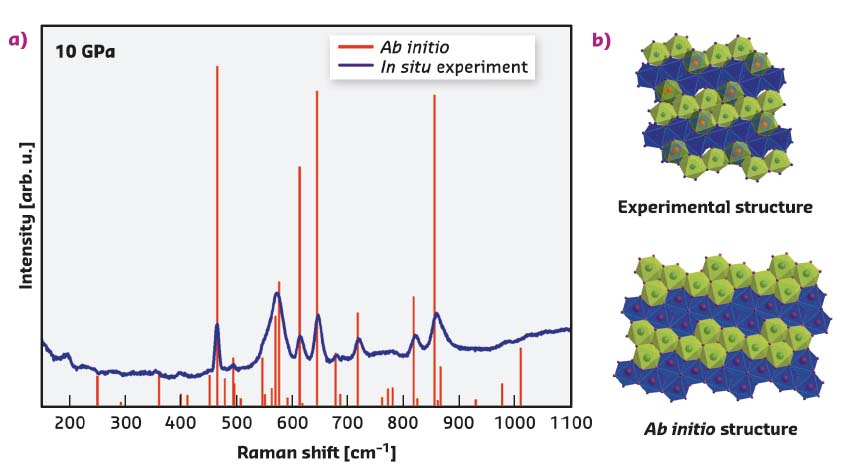
The abrupt change in Raman spectra above 10 GPa suggests that the structure of the X-I phase is quite distinct from its α- and cristobalite-II precursors (Figures 46 and 47). The polymorph cristobalite X-I belongs to the family of high-pressure silica phases comprising a distorted hexagonal close-packed array of oxygen ions, in which silicon atoms fully or partially occupy octahedral sites, being sixfold coordinated to oxygen (Figure 47). There are no other known silica polymorphs that transform to an octahedra-based structure at such low pressures upon compression at room temperature. The experimentally determined structure was then used in ab initio calculations to examine ordered structural models and to compute the Raman spectra and unit-cell parameters at various pressures (Figure 47). The theoretical study did not reveal any sign of dynamical instabilities of cristobalite X-I at pressures up to at least 100 GPa.
 |
|
Fig. 47: a) Comparison of the Raman experimental spectrum (blue continuous line) and theoretical spectra for an ordered structural model (red vertical bars). b) [010] projection of the structure of cristobalite X-I as obtained experimentally at ESRF (top), and used for model in ab initio calculations (bottom). The experimental structure contains half-occupied octahedral positions (Si3) shown by red spheres, and fully occupied octahedra (Si1 and Si2) represented by all other spheres. The blue and the green octahedra represent different levels in the structure. The theoretical structure contains 4x4 arrangement of the fully occupied octahedra. The blue and the green octahedra represent different levels in the structure. |
An increase in coordination number of silicon in cristobalite X-I from 4 to 6 occurs as low as ~10 GPa without requiring any heating, and agrees well with the results by Kubo et al. [2]. Once the thermal energy is added, cristobalite X-I transforms to the more stable arrangement – seifertite – and not to the thermodynamically stable stishovite. This could shine light onto the coexistence of α-cristobalite, formed from quenched cristobalite X-I that is only stable at high pressures, with seifertite, which is quenchable, in meteorites in which peak pressures are estimated not to have exceeded 25-30 GPa.
Based on these observations, the presence of α-cristobalite in shocked meteorites or rocks may be indicative of lower peak shock pressures.
Principal publication and authors
Compressional pathways of α-cristobalite, structure of cristobalite X-I, and towards the understanding of seifertite formation, A. Černok (a, b), K. Marquardt (a), R. Caracas (c), E. Bykova (a), G. Habler (d), H.-P. Liermann (e), M. Hanfland (f), M. Mezouar (f), E. Bobocioiu (c) and L. Dubrovinsky (a), Nat. Commun. 8, 15647 (2017); doi: 10.1038/ncomms15647.
(a) Bayerisches Geoinstitut, Bayreuth (Germany)
(b) Open University, School of Physical Sciences, Milton Keynes (UK)
(c) CNRS, Laboratoire de Geologie de Lyon, Lyon (France)
(d) Department of Lithospheric Research, University of Vienna, Vienna (Austria)
(e) Deutsches Elektronen-Synchrotron (DESY), Hamburg (Germany)
(f) ESRF
References
[1] P. Dera et al., Phys. Chem. Mineral. 38, 517-529 (2011)
[2] T. Kubo et al., Sci. Adv. 1, e1500075 (2015)



