- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Matter at extremes
- Redox-active MOF for reversible capture and release of halogens
Redox-active MOF for reversible capture and release of halogens
Chlorine and bromine are highly toxic and corrosive. A new material that could make their storage safer has been characterised by X-ray absorption spectroscopy. This material is the first metal-organic framework able to reversibly capture and release halogens.
The extreme toxicity, corrosiveness, and volatility of chlorine and bromine pose serious challenges for the safe storage and transportation of these halogens, which play a key role in the chemical industry. Solid materials capable of forming stable non-volatile compounds upon reaction with elemental halogens may partially mitigate these issues by allowing safe halogen release on demand. In this work, elemental halogens are demonstrated to quantitatively oxidise coordinatively-unsaturated CoII ions in a robust azolate metal-organic framework (MOF), Co2Cl2BTDD (1) (BTDD = bis(1H-1,2,3-triazolo[4,5-b],[4,5-i])dibenzo[1,4]dioxin), to produce stable and safe-to-handle CoIII materials, namely Co2Cl4BTDD (2) and Co2Cl2Br2BTDD (3), featuring terminal CoIII−halogen bonds.
 |
|
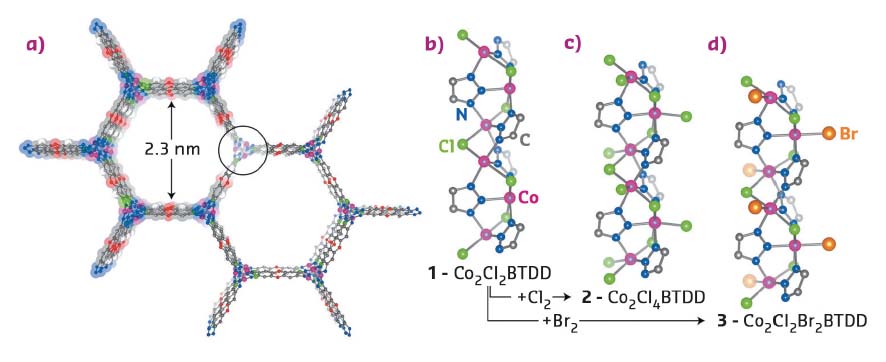
Fig. 39: Structural data. a) A portion of the structure of the parent CoII MOF Co2Cl2BTDD (1) projected along the c axis. b−d) Secondary building unit (SBU) structures and local coordination environments of the Co centres in (1), (2) and (3), respectively, as determined by NPD. |
The parent material (1) was prepared according to a previously described method [1]. Its crystal structure was confirmed by neutron powder diffraction (NPD), revealing infinite −(Co−Cl)n− chains coiled into threefold spirals and interconnected by the bis(triazolate) linkers, resulting in a honeycomb-like structure with one-dimensional channels (Figure 39a). Reaction with gaseous Cl2 or Br2 led to rapid oxidation of Co centres of the material from CoII to CoIII due to the coordination of chloride or bromide ligands, resulting in the formation of (2) or (3) respectively (Figure 39 b-d). Consequent structural changes had noticeable but rather small effects on the X-ray diffraction pattern, since the long-range order of the material was not significantly perturbed. However, the short-range local environment of Co centres was considerably altered, which made the element-selective X-ray absorption spectroscopy (XAS) measurements, performed at beamline BM23, very informative. First, ex situ XANES spectra of the parent material (1) and its oxidised counterpart (2) exhibited a pronounced chemical shift of the Co K-edge, unambiguously confirming the 2+ and 3+ oxidation state of Co centres before and after treatment, respectively (Figure 40a). EXAFS data indicated a higher coordination number for the Co-Cl shell in (2), which is direct evidence that Co centres gain one Cl extra-ligand in addition to the two Cl neighbours that are part of the framework (Figure 40 b,c).
 |
|
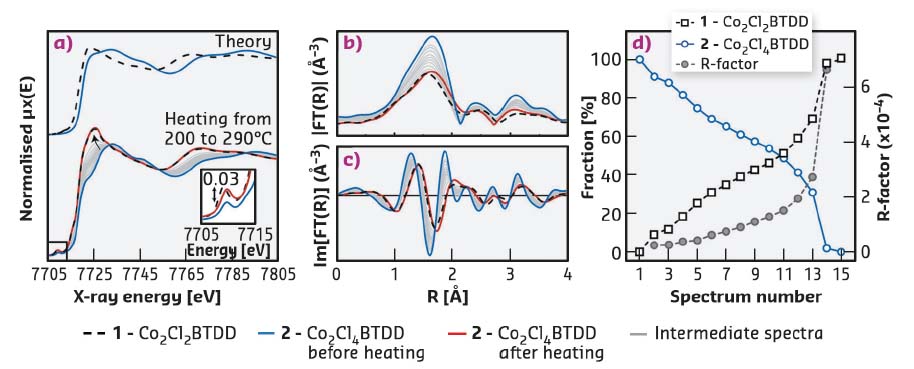
Fig. 40: Co K-edge XAS characterisation of (1) and (2). a) (top) Theoretical XANES spectra for (1) and (2) calculated from the NPD structures using the FDMNES code [2]. (bottom) Experimental XANES spectra at room temperature for (1), (2), (2) after heating, and intermediate states collected in situ in the 200-290°C range during the thermal treatment of (2). b) Magnitudes and c) imaginary parts of the phase-uncorrected Fourier transformed k2-weighted EXAFS spectra. d) Linear combination analysis of the whole series of in situ XANES spectra using the spectra of (1) and (2) at room temperature as references: relative fraction (open symbols) and fit quality factor (solid symbols). |
The reversibility of the Cl2 adsorption process was demonstrated by in situ XAS measurements performed upon heating (2) to 290°C (Figure 40 a-c). EXAFS data show a gradual decrease of the coordination number for the Co-Cl shell, while XANES spectra evidence reduction of Co centres. Importantly, both EXAFS and XANES spectra of (2) at the final stage of thermal treatment are very close to those of the parent material (1), proving the reversibility of Cl2 uptake on the local scale. Cl2 release was quantified by linear combination analysis of the in situ XANES spectra, using the spectra of (1) and (2) at room temperature as references. It indicated almost complete conversion of CoIII sites back to CoII, but the elevated R-factor at the final stages of treatment suggested the formation of a minor fraction of unreacted Co in a slightly distorted environment. Based on experimental evidence and subsequent DFT calculations, a probabilistic model of Cl release by (2) was constructed. With the only initial criterion being pairwise vicinal removal of halogens, this model predicts a residual CoIII−Cl content of 13.5%, corresponding to halogen release of 86.5% of the theoretical maximum.
These results demonstrate the ability of a CoII azolate MOF to uptake and release elemental halogens reversibly, and pave the way to the design of other porous materials geared toward the capture and storage of toxic, corrosive gases through reversible chemisorptive mechanisms.
Principal publication and authors
Reversible capture and release of Cl2 and Br2 with a redox-active metal-organic framework, Y. Tulchinsky (a), C.H. Hendon (a), K.A. Lomachenko (b,c), E. Borfecchia (d), B.C. Melot (e), M.R. Hudson (f), J.D. Tarver (f,g), M.D. Korzyński (a), A.W. Stubbs (a), J.J. Kagan (h), C. Lamberti (c,d), C.M. Brown (f) and M. Dincă (a), J. Am. Chem. Soc. 139, 5992-5997 (2017); doi: 10.1021/jacs.7b02161.
(a) Department of Chemistry, Massachusetts Institute of Technology, Cambridge (USA)
(b) ESRF
(c) IRC “Smart Materials”, Southern Federal University, Rostov-on-Don (Russia)
(d) Department of Chemistry, NIS, CrisDi, and INSTM Centre of Reference, University of Turin (Italy)
(e) Department of Chemistry, University of Southern California, Los Angeles (USA)
(f) Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg (USA)
(g) National Renewable Energy Laboratory, Golden (USA)
(h) Department of Mathematics, Weizmann Institute of Science, Rehovot (Israel)
References
[1] A.J. Rieth et al., J. Am. Chem. Soc. 138, 9401-9404 (2016)
[2] S.A. Guda et al., J. Chem. Theory Comput. 11, 4512-4521 (2015)



