- Home
- News
- General News
- Tracking live how...
Tracking live how catalysts deteriorate
09-03-2016
Catalytic converters are crucial elements in automotive industry in the process of turning harmful compounds in car exhausts into harmless compounds. Due to the harsh conditions during catalytic reactions, they deteriorate with use and time and eventually become less efficient. Researchers from Germany, the ESRF and Sweden have managed to follow in real time the degradation of catalyst nanoparticles and have worked out which of them are more resistant. Their results are published today in Nature Communications.
Share
Catalysts in a car contain noble metal nanoparticles consisting of rhodium, platinum and palladium and alloys thereof. These make the harmful molecules produced by the motor react on the surface of the converter while reaching high temperatures and reducing and oxidising atmospheres. When catalysts operate, the particle size of the noble metals increases. It is a process called sintering, which results in a decrease of the overall catalyst surface area. In the long run, this reduces the number of active sites from the surface and makes the catalyst less efficient.
Synchrotron radiation makes it possible to carry out operando experiments where scientists can monitor nanomaterials while the catalytic reaction takes place under realistic thermal and process conditions. The team, from DESY, the University of Hamburg, the University of Siegen, the ESRF and MAX IV used high-energy grazing incidence X-ray diffraction and online mass spectrometry. They combined this with the following sample design: the sample contained stripes of platinum-rhodium nanoparticles with varying composition from pure platinum to pure rhodium and a constant height. The focused high-energy beam allowed to study one stripe at a time. “The ESRF has allowed us to carry out operando experiments using a catalysis chamber at the unique high-energy beamline ID15”, explains Uta Hejral, first author of the article and from DESY and University of Hamburg. Diego Pontoni, scientist at the ESRF involved in the experiment, explains that “this kind of experiment is rather challenging because you need to control a quite complex environment characterized by different gases and high temperatures, and it is very rewarding when you see that the efforts pay off with these results”.
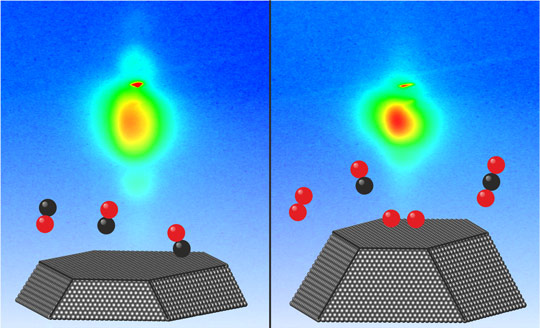
Averaged form of the platinum nanoparticles at the start of the experiments (left, surrounded by carbon monoxide) and after the fusion process (right, during reaction of oxygen and carbon monoxide into carbon dioxide). By recording 2D diffraction patterns (in the background) the change in the form of the particles can be observed in a live view.
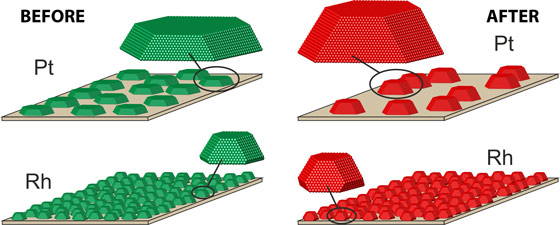
The experiments focused on the behaviour of the alloy nanoparticles during catalytic oxidation of carbon monoxide to carbon dioxide at a temperature of 550 K and near-atmospheric pressures. The results showed that platinum particles increase in height and lead to a reduction of the total particle surface coverage. “Whereas at the beginning of the experiment, the platinum particles consisted of about 15 000 atoms each, by the end they contained about 23 000. As a result of their agglomeration, the area of the carrier that was coated with platinum nanoparticles dropped from initially 50 percent to about 35 percent”, explains Hejral. This did not happen so much with the rhodium and rhodium-rich particles, which indicates that rhodium might be an important ingredient for catalyst stabilization.
While rhodium particles (lower) keep their form from the beginning (green) during the catalysis process (red), platinum particles (upper) fused together and grew substantially.
Catalysts for cars are already largely optimised based on experience, but there are still many open questions concerning the atomic scale processes that take place during reaction conditions. They need to be understood to improve the catalyst lifetimes and efficiency. “These findings have implications for the preparation of more sinter resistant particles for catalysts”, explains Andreas Stierle, professor at the University of Hamburg and DESY. “We now know that platinum-rhodium compounds work better than pure platinum, and this conclusion can be useful for car manufacturers, and could open up entirely new possibilities in the chemical industry”.
The next step for the team is now to investigate catalysts at work under reaction conditions with higher spatial resolution.
Reference
Hejral, U. et al, Nature Communications, DOI: 10.1038/NCOMMS10964.