- Home
- News
- General News
- From "alien" rivers...
From "alien" rivers to materials for technology
02-12-2016
Spanish and French scientists devote 15 years to the study of one of the most acidic rivers in the world and come up with a way of cleaning it, with a surprise in store.
Share
Tinto means “red wine” in Spanish. It is also the name of a river in the South of Spain which looks like out of this world: it is tinged red, has a very acidic pH and is extremely polluted due to more than 100 mines that line its banks. The mines date from pre-roman times and are now closed, however the minerals still leak their poison. This inhospitable river is close to another river, the Odiel, which is also heavily contaminated. The two rivers pour into the sea 30-40% of all the zinc worldwide that reaches the oceans, as well as 10% of all the copper. In the past, researchers at NASA have studied the Tinto River as a model for the extreme conditions present in Mars. Citizens in nearby towns are not allowed to use the water from these rivers in any way.
The phenomenon responsible for the colouring of Tinto River waters is known as acid mine drainage (AMD). AMD is the result of pyrite oxidation, a chemical reaction that releases acidity into the environment. When these acid waters strike natural waters from adjacent rivers, natural nanoparticles of iron and aluminum oxy-hydroxides are formed, resulting in the beautiful colours that decorate the otherwise arid landscape. But a lot more is hidden behind the spectacular colours: these natural nanoparticles act as scavengers of toxic elements such as arsenic and selenium, and they concentrate Rare-Earth elements (REE), which are also known as ‘critical materials’ for their use in technological applications.
|
The Río Tinto, with different tinges depending on the Ph of the water. |
In the last 15 years, a team from the Universidad of Huelva, which is very close to the site, the Institute of Environmental Assessment and Water Research (IDAEA-CSIC) from Barcelona, the ISTerre (CNRS, Université Grenoble-Alpes) in Grenoble, and the ESRF, has tried to get a detailed nanoscale picture of the different geochemical processes underlying AMD. The result: experiments on seven beamlines, more than a dozen papers, clean water and the side-effect production of REEs. “It is a very exhaustive story”, explains Alex Fernández-Martínez, a member of the team from the ISTerre (CNRS). There are two PhD students currently working on the subject and there have been twelve since the beginning of the project. This week, their latest results have been published in the journal Environmental Science and Technology.
From an analysis of the nanomaterials in the rock …
It all started when researchers studied the nanominerals that developed when the rock in the area (pyrite) comes into contact with rain. “Our first experiments were devoted to learning how naturally-occurring pollutants are transported by the iron and aluminum nanominerals in the acid waters”, says Rafael Perez-Lopez, from the University of Huelva. Scientists published the structures of schwertmannite and basaluminite, two of the more relevant nanominerals, over the last six years. The atomic-level information of the structure of these disordered phases was provided by Pair Distribution Function (PDF) experiments at the ESRF's ID15, ID31 and ID22 beamlines, in close collaboration with Agnieszka Poulain (ESRF). Further experiments at BM25 using X-ray absorption (EXAFS) gave information about the location of the contaminants in the structure of these nanominerals: “This was a crucial step for the design of effective remediation strategies”, says Perez-Lopez.
The team used an array of techniques at the ESRF for this research, from X-ray absorption spectroscopy, to small-angle X-ray scattering, diffraction and PDF analyses in different beamlines. It is quite exceptional that a same subject of research can be studied in such a range of beamlines.
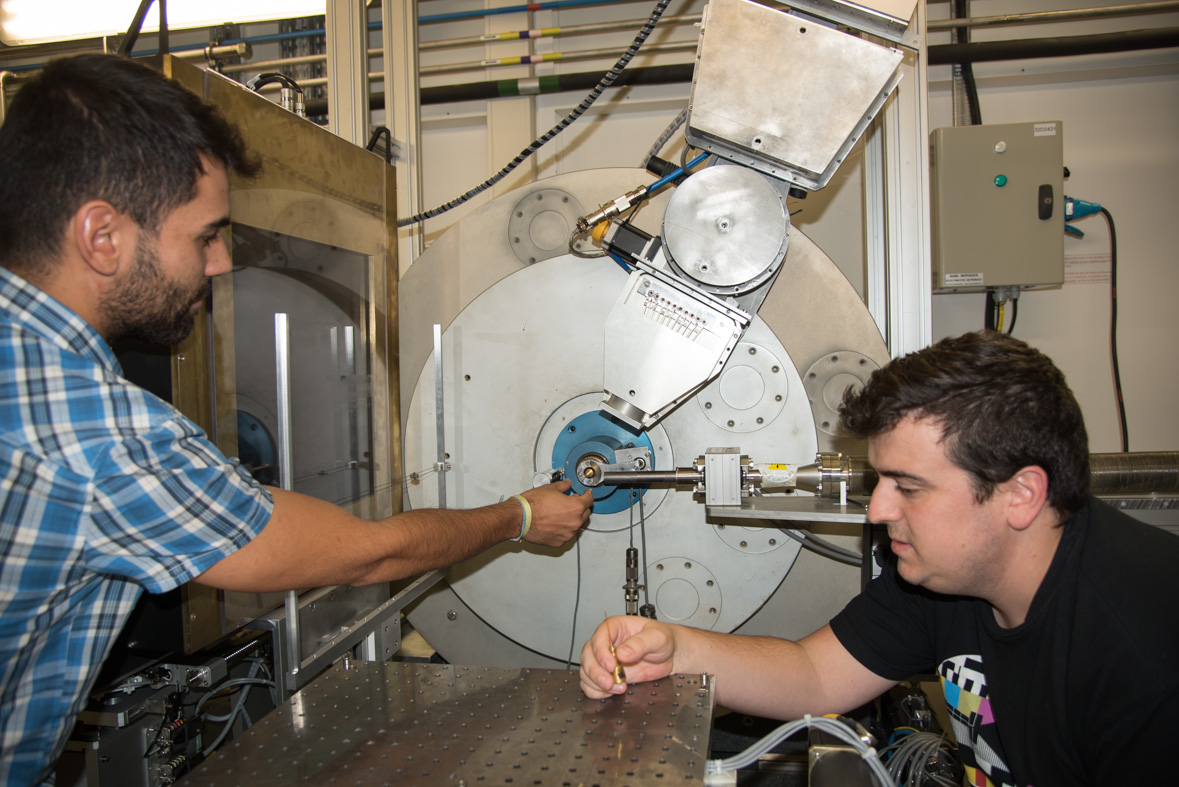 |
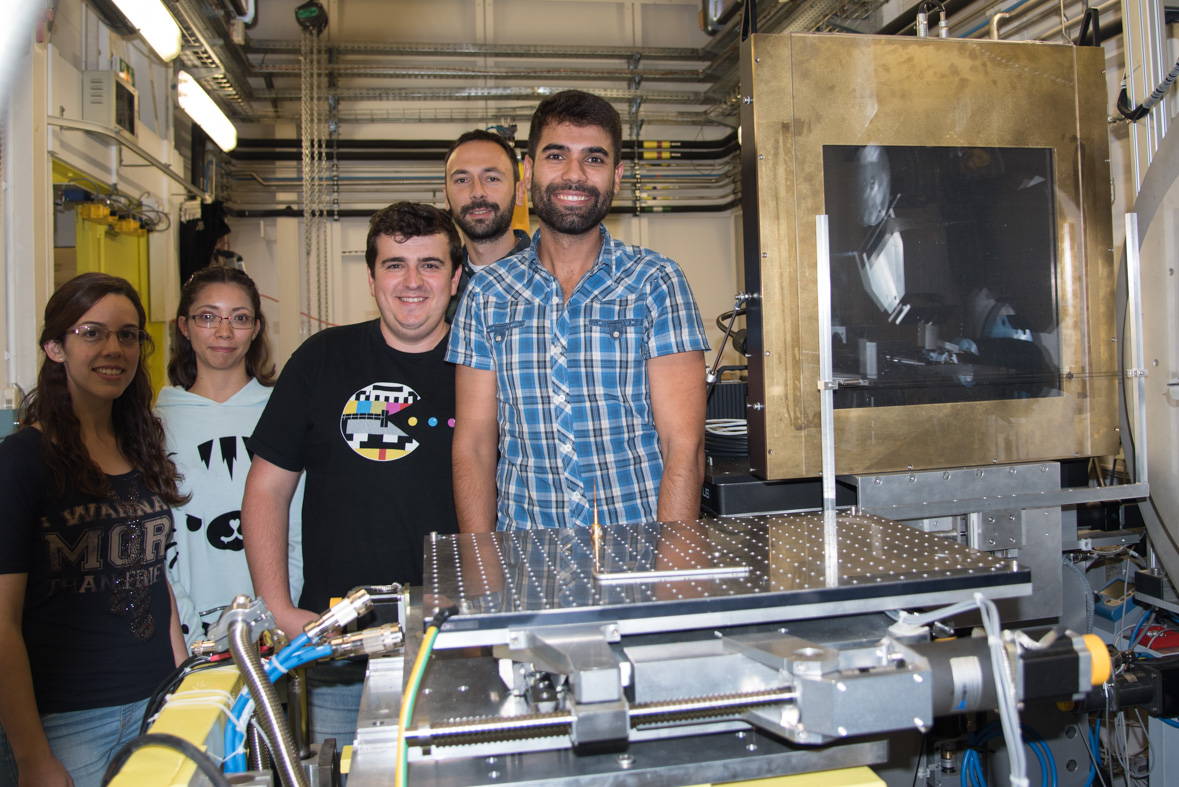 |
|
The team during their last experiment at the ESRF, on ID22. Second picture, from left to right: Alba Lozano, Ingrid Nayeli, Pablo Cruz, Álex Fernández-Martínez, Sergio Carrero. Credits: C.Argoud/ESRF. |
|
They also got insight into the formation of minerals using BM26, and about their texture using ID11. In situ small-angle X-ray scattering experiments showed the different steps of formation of the aluminum nanoparticles, mimicking the real chemical conditions of the rivers. X-ray diffraction microtomography was used to study the textural properties of schwertmannite, the iron oxide nanoparticles. The findings from all these experiments at the ESRF result in a complete characterization of the mineralogy and geochemistry of the rivers. “It is interesting to see that the same processes that we found in nature are nowadays used in laboratory syntheses of nanomaterials. We can learn a lot from nature”, says Sergio Carrero-Romero, a PhD student working between Huelva and Grenoble.
… to hitting the jackpot
 |
|
Alba Lozano, from the team, prepares a sample at the ESRF. Credits: C.Argoud/ESRF. |
In parallel to this research, scientists at the University of Huelva and the IDAEA were trying to figure out how to decontaminate the water from the two rivers to make it useable by the population. They came up with the idea of building big pools in which they could neutralize the acidity using dissolving limestone. But they were in for a surprise: “This is a quite straightforward system, we started to test it in the lab in 2005, and we implemented it in the field in 2011. What we didn´t expect was what the solid residues contained” explains Carlos Ayora, from IDAEA. When they analysed the precipitates on ID21 and BM25, they “hit the jackpot”. Using the techniques of X-ray micro-fluorescence and X-ray absorption spectroscopy, they found high concentrations of REEs associated with the structure of the aluminum nanoparticles. REEs are used routinely in technology such as mobile phones or batteries. This was a real surprise as they have an important economic value. Today, China has the monopoly of REE thanks to its multitude of mines, and it supplies the rest of the world. “Coming across such precious materials unexpectedly was a game changer. We could potentially exploit this to fund at least the purifying process, so it wouldn’t cost money and the population in the neighbouring area could use the water”, explains Jose Miguel Nieto, from the Universidad of Huelva.
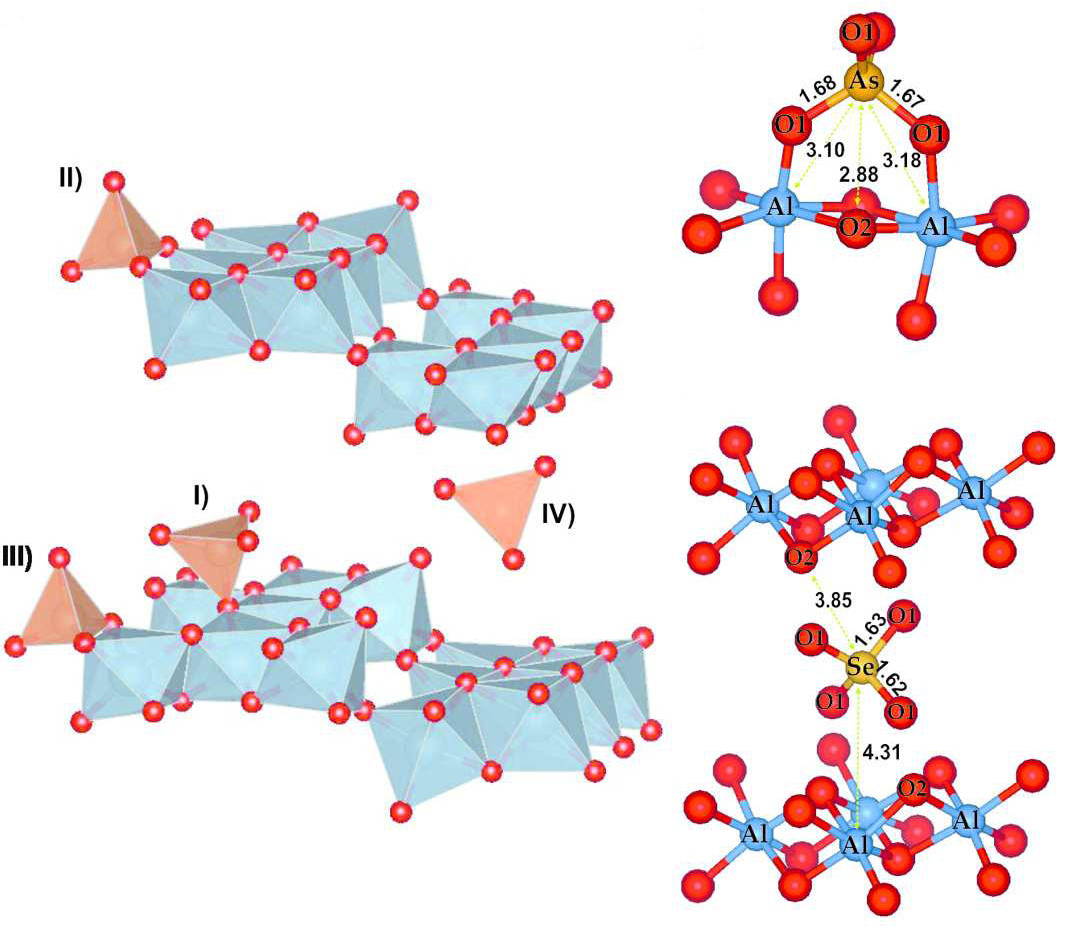 |
Models of basaluminite (left side of picture) structure reported by Farkas and Pertlik (1997) doped with arsenate or selenate located in: I) monodentate inner-sphere, II) bidentate inner-sphere, III) bidentate mononuclear inner-sphere and VI) outer-sphere theoretical position; and the most probable structural location of arsenate (top right) and selenate (middle and bottom right) onto basaluminite. The signal intensity was normalized by the maximum of Al-O distance. Reprinted with permission from Carrero S et al, (2016) Arsenate and selenate scavenging by basaluminite: Insights into the reactivity of aluminum phases in acid mine drainage, Environ. Sci. Technol., DOI: 10.1021/acs.est.6b03315. Copyright 2006 American Chemical Society. |
Many times the academic work doesn’t go beyond the University walls. In this case, however, a multidisciplinary team has managed to make a change for some citizens and the analysis at the ESRF have contributed to a great extent. As Alejandro Fernández-Martínez says: "It's been a really great experience working on the Rio Tinto. This work has combined field and lab-based research, including many data collected at the ESRF. Thanks to this multi-scale approach, we've been able to get out and offer solutions to a real ecological problem. What's more we got the added bonus of being able to auto-finance the decontamination, so thus doubly helped the population around the river".
Text by Montserrat Capellas Espuny
References
Ayora C., et al, (2016) Recovery of Rare Earth Elements and Yttrium from Passive-Remediation Systems of Acid Mine Drainage. Environmental science & technology, 50: 8255-8262
Carrero S., et al,(2015) The potential role of aluminium hydroxysulphates in the removal of contaminants in acid mine drainage. Chemical geology, 417: 414-423
Perez-Lopez, R, et al, (2011) Mineralogy and Geochemistry of Zn-Rich Mine-Drainage Precipitates from an MgO Passive Treatment System by Synchrotron-Based X-ray Analysis. Environmental Science &Technology, 45: 7826-7833
Perez-Lopez R, et al, (2011) Synchrotron-based X-ray study of iron oxide transformations in terraces from the Tinto-Odiel river system: Influence on arsenic mobility. Chemical Geology, 280: 336-343
Fernandez-Martinez A., et al, (2010) The structure of schwertmannite, a nanocrystalline iron oxyhydroxysulfate American Mineralogist, 95, 1312-1322.
Top image: Río Tinto, in the south of Spain.



