- Home
- News
- Spotlight on Science
- Iron electronic...
Iron electronic state and the electrical conductivity of the Earth’s lower mantle
19-11-2013
Electrical conductivity profiles of the Earth’s mantle provide an important complement to seismic profiles for deducing the nature of the Earth’s interior through comparison with electrical conductivity measurements carried out in the laboratory at extreme pressures and temperatures. A novel method, the synchrotron Mössbauer source, developed at beamline ID18, is highly sensitive to changes in the electronic structure of iron (oxidation and spin state) and enables the interpretation of laboratory conductivity measurements. Electrical conductivity is strongly influenced by iron oxidation and spin state and provides one more example of how small changes in electronic structure can cause large changes in physical properties, bringing us one step closer to understanding how the Earth’s internal engine works.
Share
Much of our knowledge of the Earth’s interior comes from comparison of laboratory measurements of physical properties of mantle minerals with data from bulk geophysical methods. Traditionally seismology has provided the best indications of the internal structure of the Earth; however electromagnetic methods can be used to construct electrical conductivity profiles, which are more sensitive to temperature and composition variations.
Iron is the most abundant element inside the Earth and variations in pressure or temperature can cause changes in the number of electrons (oxidation state) or in the distribution of electrons (spin state), which can affect electrical conductivity. The iron atoms in the Earth’s most abundant phase, (Mg,Fe)(Si,Al)O3 perovskite, are known to exist in two different oxidation states (ferrous iron and ferric iron), and both oxidation states have the possibility to undergo a spin state transition at high pressure. Deducing the nature of iron spin transitions in (Mg,Fe)(Si,Al)O3 perovskite is challenging due to the numerous combinations of oxidation and spin state in the two crystallographic sites of its crystal structure.
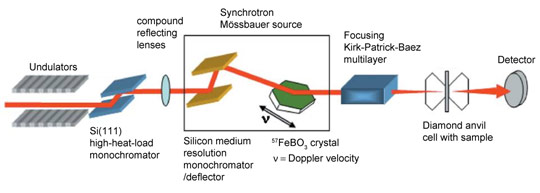
Energy-domain 57Fe Mössbauer spectroscopy can provide an unambiguous resolution of all hyperfine parameters which can be used to infer spin states; however high-pressure measurements using conventional radioactive point sources require extremely long counting times. Third-generation synchrotron sources offer a solution in the form of time-domain Mössbauer spectroscopy (i.e., nuclear forward scattering); however, this method is not well suited to materials with a large number of components because of the resulting complex spectra. To solve this problem, we have developed an energy-domain synchrotron Mössbauer source (SMS) [1] at beamline ID18, the Nuclear Resonance Beamline, which offers a number of advantages over conventional Mössbauer spectroscopy: high brilliance and a nearly fully resonant emitted beam that can be focused to a spot of only a few micrometres in diameter. Figure 1 displays a sketch of the SMS facility.
 |
|
Figure 1. Sketch of the SMS facility at beamline ID18. |
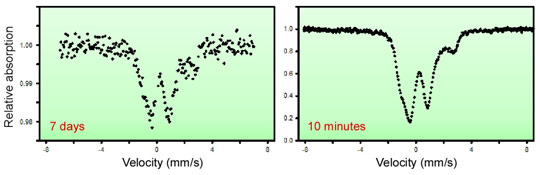
Figure 2 illustrates the advantage of the SMS spectroscopy compared to conventional Mössbauer spectroscopy using a radioactive source. This device is now available for the users programme at beamline ID18.
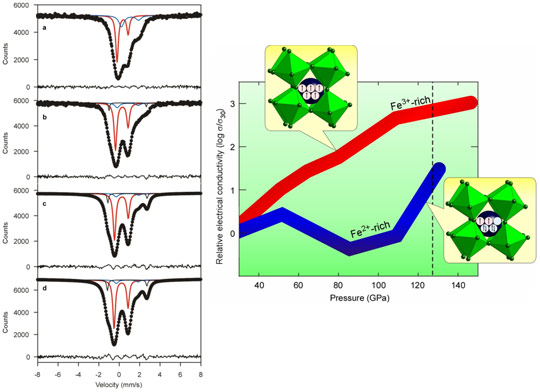
In this study we used SMS spectroscopy to study the effect of pressure on the hyperfine interactions of (Mg,Fe)(Si,Al)O3 perovskite compressed and heated using a laser-heated diamond anvil cell. We collected spectra up to 122 GPa. A representative set of SMS spectra is shown in Figure 3 left panel. We found that no spin transition occurs in ferric iron (Fe3+ ) at lower mantle conditions, contrary to previous claims. Instead, ferrous iron (Fe2+) likely undergoes a partial spin transition to an intermediate spin state, suggesting that the previously observed sharp drop in electrical conductivity of (Mg,Fe)(Si,Al)O3 perovskite [2] is due to a spin transition of ferrous iron. To test this hypothesis, we measured the high-pressure high-temperature electrical conductivity of (Mg,Fe)(Si,Al)O3 perovskite containing predominantly ferric iron, and observed no drop in electrical conductivity (Figure 3).
Temperature and total iron content were previously considered to have the greatest influence on lower-mantle electrical conductivity profiles, but our data show that the ferric to ferrous iron ratio can also affect conductivity significantly at pressures corresponding to the middle part of the lower mantle. Combined with results from our other studies that measured the effect of iron on the elastic wave velocities of (Mg,Fe)(Si,Al)O3 perovskite, our new data can provide improved constraints for joint inversion of electromagnetic and seismic data, potentially yielding a new probe of heterogeneity in the lower mantle.
Principal publication and authors
Effect of iron oxidation state on the electrical conductivity of the Earth’s lower mantle, V. Potapkin (a,b), C. McCammon (b), K. Glazyrin (b), A. Kantor (a,b), I. Kupenko (a,b), C. Prescher (b), R. Sinmyo (b), G.V. Smirnov (c), A.I. Chumakov (a,c), R. Rüffer (a), L. Dubrovinsky (b), Nature Communications 4, 1-6 (2013).
(a) ESRF
(b) Universität Bayreuth, Bayreuth (Germany)
(c) National Research Center ‘Kurchatov Institute’, Moscow (Russia)
References
[1] V. Potapkin, A.I. Chumakov, G. V. Smirnov, J.-P. Celse, R. Rüffer, C. McCammon and L. Dubrovinsky, J. Synchrotron Radiat. 19, 559 (2012).
[2] K. Ohta, K. Hirose, M. Ichiki, K. Shimizu, N. Sata and Y. Ohishi, Earth Planet. Sci. Lett. 289, 497 (2010).
Top image: Synchrotron Mössbauer source spectra provided electron configurations for (Mg,Fe)(Si,Al)O3 perovskite under extreme conditions.