- Home
- News
- Spotlight on Science
- Studying metal alloy...
Studying metal alloy solidification using X-ray radiography and machine learning
10-07-2018
Solidification plays a critical role in controlling the final structure and properties of most metallic components. X-ray radiography was combined with machine learning to investigate the solidification behaviour of Al-Cu alloys and the effect of grain refiners.
Share
The final properties of most cast metallic components are dictated by the microstructure developed during solidification which is often impossible, impractical or expensive to modify during down-stream processes. This microstructure is the result of phase-transformation phenomena occurring during the liquid-to-solid transition and, as in many phase-transformation phenomena, is controlled by the nucleation and growth of the different phases [1]. When cooling a molten metal below its melting point, solidification is initiated by the nucleation of the first solid crystals, usually close to the melting point of the alloy, and proceeds in a continuous process of nucleation and growth until all the liquid is consumed and the material transforms into a solid. It is well known from manufacturing experience that the size and shape of the various phases can be controlled by promoting or suppressing their nucleation. The best-known example is inoculation in the aluminium industry, where insoluble micrometre-sized grain-refining particles are added to alloys just before casting to promote the easy nucleation of refined and isotropic aluminium-rich crystals, with beneficial effects on final mechanical properties and material consistency.
Although nucleation theory has been developed since the 1950s [2], experimental studies on metal alloys have been challenging as nucleation take place over short time scales and at the nano- to microscale. By using the outstanding imaging capabilities of beamline ID19, a comprehensive in situ study of nucleation in aluminium alloys has been carried out. The work involved the collection of 128 X-ray radiography video sequences of the crystallisation of solidifying Al-Cu alloys (inoculated with TiB2 particles) over a wide range of conditions. Each experiment consisted of melting a thin foil sample in a bespoke furnace and then using controlled cooling to induce crystallisation while recording radiographic videos of the transformation. Critically, a machine learning algorithm was developed to identify individual crystals in the video sequences. The algorithm was trained using a subset of 7 out of 128 sequences and was capable of counting the number of grains in each individual frame, locate their position, track their movement and growth and measure their nucleation undercooling.
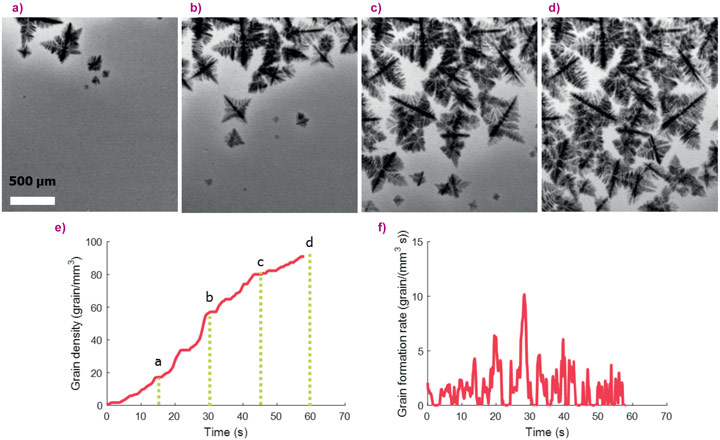
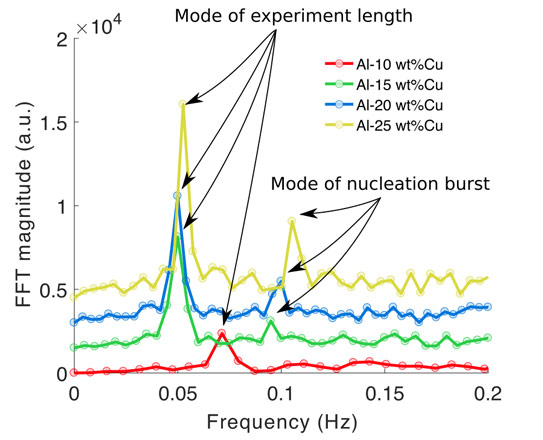
Figure 1(a-d) is a typical image sequence showing the progressive nucleation and growth of Al crystals (grains) from the liquid phase. Figure 1e shows the density of grains in each frame measured using the machine learning algorithm and the label refers to the frames shown in a to d. The data revealed that nucleation took place mainly in bursts, which is evident in the formation rate plotted against time in Figure 1f. The machine learning analysis was applied to the entire video database and almost 14,000 separate nucleation events were detected. The analysis of the combined data revealed that nucleation occurred in repeated bursts, the strength of which related to the alloy composition as shown in Figure 2 where the FFT of the nucleation rate for four different alloys shows their characteristic nucleation frequencies. The origin of these bursts is likely due to the accumulation of solute ahead of the solid-liquid front which suppresses “easy” nucleation at low undercooling and promotes more “difficult”, but more intense, nucleation at higher undercooling, making the overall nucleation process more productive. These experimental findings are consistent with the ideas proposed in the interdependence theory [1] – that the solute field in the liquid around an assemble of crystals affects the ability to form further crystals – but adds new understanding that the new crystals form in distinct bursts rather than semi-continuously.
 |
|
Figure 2. The frequency spectrum of the crystal formation rate as a function of time for four Al-Cu alloys cooled at 1.5 K/s. FFT, fast Fourier transform; a.u., arbitrary units. |
Principal publication and authors
Crystal nucleation in metallic alloys using X-ray radiography and machine learning, E. Liotti (a), C. Arteta (b), A. Zisserman (b), A. Lui (a), V. Lempitsky (c) and P.S. Grant (a), Sci. Adv. 4, eaar4004 (2018). doi: 10.1126/sciadv.aar4004.
(a) Department of Materials, University of Oxford (UK)
(b) Department of Engineering Science, University of Oxford (UK)
(c) Skolkovo Institute of Science and Technology, Moscow (Russia)
References
[1] D. StJohn, M. Qian, M.A. Easton and P. Cao, The interdependence theory: the relationship between grain formation and nucleant selection, Acta Materialia, 59, 4907-4921 (2011).
[2] A.L. Greer, Overview: Application of heterogeneous nucleation in grain-refining of metals. J. Chem. Phys. 145, 211704 (2016).