- Home
- News
- Spotlight on Science
- Probing protein...
Probing protein dynamics in concentrated protein solutions using X-ray photon correlation spectroscopy
24-01-2023
The unique properties of ESRF’s Extremely Brilliant Source (EBS) were exploited at beamline ID10 to probe protein dynamics in concentrated protein solutions. The results support a postulated analogy between protein and colloid dynamical arrest.
Share
Macromolecular crowding is a phenomenon occurring inside all living cells, whereby very high concentrations of macromolecules such as proteins in an intracellular environment interact differently compared to lower concentrations, altering the dynamics of molecular interactions, chemical reactions and protein diffusion. In order to investigate the influence of crowding on protein diffusion, an experiment was performed at the beamline ID10 to study the microscopic dynamics of different concentrations of protein solutions. As a model system, concentrated solutions of the eye lens protein α-crystallin was used, which, in structural and rheological terms, follows the classic behaviour of hard sphere colloids (i.e. insoluble particles suspended in another substance as a mixture) under these conditions [1].
Processes such as the diffusion of proteins take place at length scales from tenths of Ångstrom to nanometres and on time scales from nanoseconds to seconds. While time scales below microseconds can be covered by neutron spin echo spectroscopy, the only technique that can measure slower dynamics in concentrated protein solutions at the particle length scale is X-ray photon correlation spectroscopy (XPCS).
However, until recently, weak scattering power and radiation damage to proteins made the application of XPCS difficult. To overcome the issue of radiation damage, a large beam with a high degree of coherence was used at the ESRF’s new Extremely Brilliant Source (EBS). A dedicated experimental and analysis strategy was applied to overcome the radiation damage and extract the intrinsic dynamics in the solution from those induced by the beam.
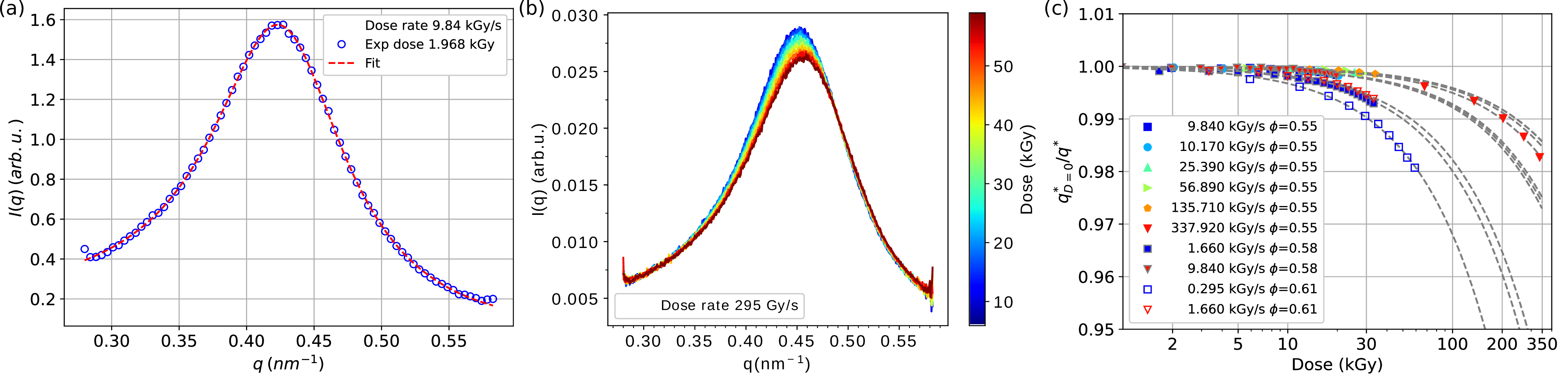
The conventional approach to measuring proteins with X-rays is to limit the radiation dose to below the critical value where structural changes can be observed. First, the effect of the X-rays on the structure of α-crystallin solutions was investigated using small angle X-ray scattering (SAXS) in order to find the critical dose (Figures 1a and 1b). By analysing the SAXS curves as a function of dose (Figure 1c), it was estimated that the critical dose limit was around 10 kGy.
Click to enlarge
Fig. 1: Effect of X-rays on the structure of α-crystallin solutions. a) Measured scattering intensity of the concentrated α-crystallin solution at a volume fraction f=0.55, where the red dashed line is a fit using a polydisperse hard sphere model. b) Evolution of the scattering intensity with the deposited dose. c) Inverse of the relative peak position as a function of deposited dose and dose rate for different volume fractions. The guides to the eye (grey dashed) are exponential decays.
Next, XPCS measurements were carried out at ID10 to probe the dynamic properties of the protein solutions. In particular, the effect of the different protein concentrations on cage relaxation was investigated. In a proposed analogy with colloid behaviour such as glass formation, the ‘cage’ effect refers to when the diffusion of individual particles through a dense liquid is restrained by entrapments in cage-like structures formed by neighbouring particles, leading to dynamic arrest (and glass transition) [2]. Cage relaxation is when an opening forms in the cage to allow the passage of a particle.
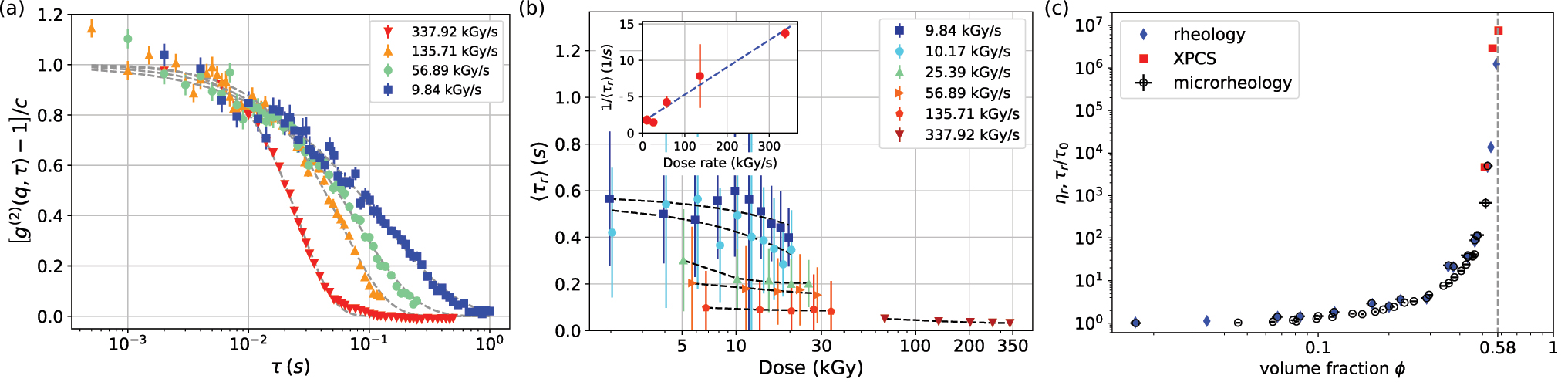
Surprisingly, the XPCS measurements showed that the cage relaxation time became faster with the increasing X-ray dose rate, even if the total dose remained below the critical value (Figure 2a). Careful analysis of the results obtained for different protein concentrations revealed a complex dependence of the relaxation time on dose and dose rate (Figure 2b), evidencing a radiation-induced dynamics also previously seen in glasses [3]. A likely explanation is that radiation causes the formation of covalent bonds between individual α-crystallin proteins. This cross-linking leads to the opening of a local void in the nearest-neighbour cages of the crowded protein solutions, into which the surrounding proteins can relax with a locally directed motion.
Click to enlarge
Fig. 2: Dynamic properties of a-crystallin solutions. a) Normalised intensity correlation functions for f=0.55 as a function of dose rate. b) Average relaxation time is decreased by both dose and dose rate. Inset: the relaxation rate as a function of dose rate. c) Relative zero-shear viscosity of a-crystallin solutions as a function of volume fraction obtained from rheology and micro-rheology, and normalised relaxation time obtained from XPCS.
It is therefore expected that the observed relaxation is due to both radiation-induced dynamics and an intrinsic cage relaxation mechanism due to diffusive (or sub-diffusive) motion. Using a dedicated experimental and analysis protocol to separate the two [4], the intrinsic dynamics from linear extrapolation to zero dose rate (inset in Figure 2b) was estimated. The results obtained for the intrinsic ‘long-time cage diffusion’ relaxation times agree with macroscopic measurements of the relative zero-shear viscosity (Figure 2c).
In conclusion, XPCS was used to reveal information on the physical mechanism behind dynamical arrest on the nearest-neighbour length scale that, with previous studies, completes the missing link in the colloidal picture of dynamical arrest of α-crystallin, supporting the postulated physical analogy between globular proteins and hard colloids [5]. These results also provide a methodological feasibility test, showing that dose rate, as well as dose, are the relevant parameters to control in XPCS measurements. Given the enormous improvements of coherent beam characteristics thanks to EBS, XPCS is thus a highly promising approach for future in-depth characterisation of local dynamics in biological and soft materials.
Principal publication and authors
Probing Cage Relaxation in Concentrated Protein Solutions by X-Ray Photon Correlation Spectroscopy, Y. Chushkin (a), A Gulotta (b), F. Roosen-Runge (b,c), A. Pal (b), A. Stradner (b,d), P. Schurtenberger (b,d), Phys. Rev. Lett. 129, 238001 (2022); https://doi.org/10.1103/PhysRevLett.129.238001
(a) ESRF
(b) Division of Physical Chemistry, Lund University, (Sweden)
(c) Department of Biomedical Science and Biofilms Research Center for Biointerfaces, MalmӧUniversity,(Sweden)
(d) LINX, Lund University, (Sweden)
References
[1] G. Foffi et al., PNAS 111, 16748 (2014).
[2] P.N. Pusey & W. Van Megen, Phys. Rev. Lett. 59(18), 2083 (1987).
[3] B. Ruta et al., Sci. Rep. 7, 3962 (2017).
[4] Y. Chushkin, J. Synchrotron. Radiat. 27, 1247 (2020).
[5] A. Stradner & P. Schurtenberger, Soft Matter 16, 307 (2020).
| About the beamline: ID10 |
|
ID10 is a soft interfaces and coherent scattering beamline composed of two end-stations. End-station EH1 is for high-resolution X-ray scattering and surface diffraction on liquid and solid interfaces, combining multiple techniques in a single instrument. This allows investigation of the structure and self-organisation processes at surfaces, interfaces and in thin films both in-plane and normal to the film. End-station EH2 is for coherent small-angle X-ray scattering, X-ray photon correlation spectroscopy, which allows the study of slow in-equilibrium and out-of-equilibrium dynamics in disordered or modulated materials on timescales beyond the reach of the inelastic (X-ray or neutron) techniques, and coherent diffraction, allowing for the high-resolution imaging of biomineral hierarchical structures, porous semiconductor materials, mineral nanocrystals and nanostructures, biological cells, etc. |