- Home
- News
- General News
- ESRF lightsource...
ESRF lightsource helps tailoring new treatments against asthma
01-08-2007
PRESS RELEASE- Researchers from Sweden and France have deciphered the crystal structure of a human membrane protein which has a major influence on the development of asthma. They used the highly automated ESRF macromolecular crystallography experimental stations (beamlines) to determine the structure of this pharmaceutically important protein, only the third human membrane protein to be solved. The scientists now believe that their work will enable the development of new and better therapeutic drugs targeting inflammation of the respiratory tract.
Share
Asthma affects 300 million people worldwide and, according to World Health Organization, it killed 255 000 people in 2005. Asthma attacks are caused by an acute inflammatory reaction in the airways, a reaction that is largely due to actions of LTC4 synthase (an enzyme which catalyzes a synthesis process). For this reason asthma medicines often aim to block the downstream effects of LTC4 synthase.
However, there is a need for new pharmaceutical alternatives, since not all patients respond to the existing medicines. Thanks to the research partly carried out at the European Synchrotron Radiation Facility (ESRF), it is now possible to tailor new molecules that can block the LTC4 synthase. “I believe this breakthrough will speed up drug discovery against this disease”, explains Andrew McCarthy, one of the researchers in the team.
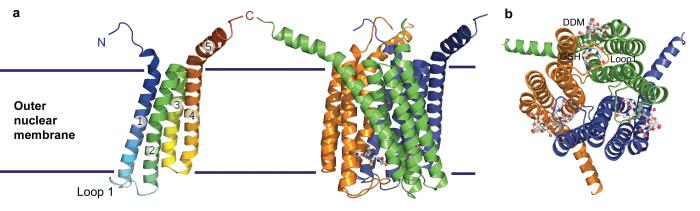
Scientists from the Karolinska Institute and the University of Stockholm in Sweden, together with colleagues from the European Molecular Biology Laboratory in France have solved the three dimensional structure of the LTC4 synthase at 2.0 angstrom resolution. The protein has three identical subunits, each consisting of four helical structures that span the membrane. The structure finally allows the exact position and characteristics of the active sites, where activating or blocking molecules can bind, to be identified.
The study of this protein at the ESRF proved a challenge for the team. The crystallization of membrane proteins is quite a complex process and even more challenging if they are human. So far only three human membrane proteins have been structurally characterized. The team carried out experiments at the ESRF to screen crystals several times before finally being able to determine the 3D structure.
Membrane proteins- an open door to new pharmaceuticals
The new results can lead the way for the development of new and more effective medications against other diseases. Some 40% of the proteins of interest for pharmaceutical developments are membrane proteins.
Until now detailed structural information on these proteins has been absent, and therefore it has been difficult to fully understand their function. The present study is likely to lead the way for the determination of structures of other human membrane proteins. Unravelling the secrets of more membrane protein structures will help understand fundamental processes that take place in the cell membranes.
Reference
"Structural basis for synthesis of inflammatory mediators by human leukotriene C4 synthase", Martinez Molina D, Wetterholm A, Kohl A, McCarthy AA, Niegowski D, Ohlson E, Hammarberg T, Eshaghi S, Haeggström JZ, Nordlund P, Nature AOP 15 july 2007.
For more information, please contact Montserrat Capellas ( email or +33 (0)476882663).