- Home
- News
- General News
- I can sense your...
I can sense your heartbeat
11-01-2011
MRC Cambridge takes "near-action shots of vital proteins" at the ESRF with the potential for pharmaceutical companies to improve drug design, says Nature news.
Share
G protein-coupled receptors (GPCRs) are a large family of proteins with up to 800 different types in humans. GPCRs are membrane proteins that bind specifically a large number of activating molecules and thereby convey sensory information or changes in physiological state between cells. Their structures have been the subject of intensive, competitive research and until recently were used as the paradigm for an almost intractable structural biology problem. Although elusive, the structures of several GPCRs have been elucidated in the past years, providing considerable insight into the function and specificity of these proteins. A key issue that remains is the mechanism of activation of G proteins upon binding of the activating molecule, an agonist, to the receptor.
An important class of GPCRs are the β-adrenergic receptors. The β1AR and β2AR are responsible amongst other things for the activation of the G protein that signals increases in the force of either cardiac contraction or vasodilation respectively. These are the subjects of three papers, along with a “News and Views” highlight, in the 13 Jan 2011 issue of NATURE. One of the three papers involved a long series of experiments at the ESRF.
In order to attempt to unravel how subtle structural changes induced by agonist binding are converted into G protein activation, Tony Warne and colleagues from the MRC Laboratory of Molecular Biology in Cambridge (UK) solved four structures of the β1AR with either agonist or partial agonist bound. From these structures they were able to identify the interactions that appear to be crucial in initiating the transmission of the signal to the G protein target.
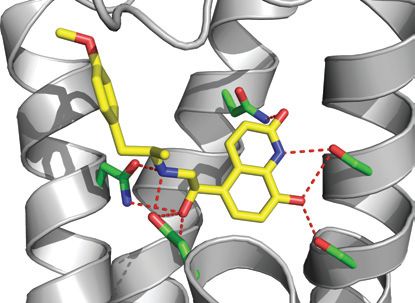 |
The binding of the agonist carmoterol in the structure of the β1-adrenergic receptor. The orientation is the same as in the image above. Credit T. Warne et al./Nature Publishing Group |
|---|---|
The structural biology of GPCRs is made problematic by the small size and the low intrinsic order of the available crystals, requiring the screening of many hundreds of crystals in order to obtain useful data sets. A key element in helping make this possible has been the availability at the ESRF of high performance micro-focus X-ray beams combined with state of the art sample automation and tracking. Obtaining the necessary data required multiple visits to the ESRF for experiments taking place on ID23-2 over as long as six months. Lead author Tony Warne comments: “Once again the use of a microfocus beamline was essential; all datasets were collected from single crystals, many wedges were collected at different positions, and finally up to 13 of the best wedges of data were merged.”
Reference: Warne, T. et al. Nature 469, 241–244 (2011).
To read an interesting article in nature news dated 13 January 2011 on how this work relates to the other papers published in that issue of Nature, and to the development of new drugs, go to:
http://www.nature.com/news/2011/110112/full/news.2011.13.html
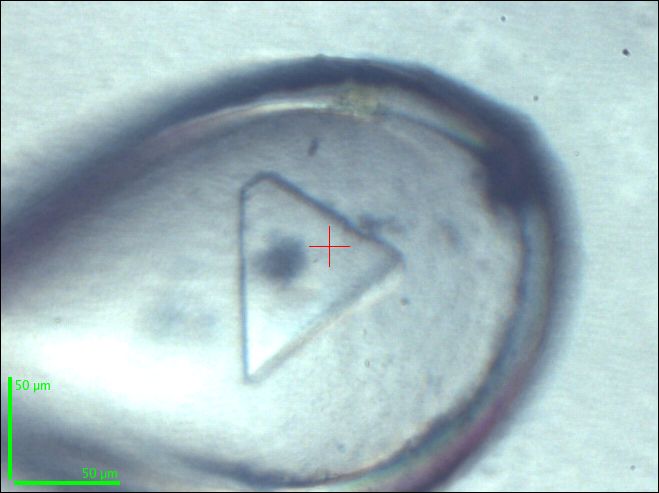 |
A cryo-cooled crystal of β1AR with the partial agonist dobutamine bound which was used for data collection at the ESRF microfocus beamline ID23-2. Credit T. Warne |
|---|---|
Top image: Structure of the β1-adrenergic receptor bound to an agonist, in cartoon representation with the intracellular side at the bottom of the figure. The ligand carmoterol is shown as a space filling model. Credit T. Warne et al./Nature Publishing Group



