- Home
- News
- General News
- X-rays help advance...
X-rays help advance the battle against heart disease
04-10-2011
Scientists from Imperial College London and Diamond Light Source have revealed the structure of a cholesterol-lowering drug target. Published in the journal Nature, this finding could lead to much more effective drugs to tackle high cholesterol levels, a condition that increases the risk of heart disease.
Share
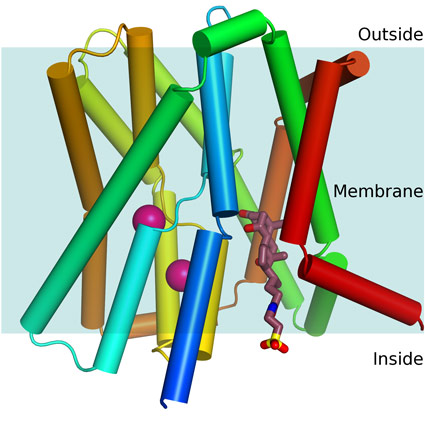
The researchers from Imperial College London used intense X-rays, generated by the Diamond synchrotron and the European Synchrotron Radiation Facility (ESRF), to determine for the first time the structure of a bacterial homologue of the apical sodium dependent bile acid transporter (ASBT) protein, a target for hypercholesterolemia drugs since it can affect the level of cholesterol in the blood.
In the liver, cholesterol makes bile acids which are used in the intestine to absorb fat. These bile acids are then reabsorbed by ASBT to be transported to the liver and recycled. It is known that by blocking ASBT, bile acid levels returning to the liver are lowered, the liver therefore converts more cholesterol into bile acids, which lowers the level of cholesterol in the blood.
Professor So Iwata, David Blow Chair of Biophysics at Imperial College London, BBSRC Fellow and Director of the Membrane Protein Laboratory (MPL) at Diamond, said: “There are currently a number of existing ASBT inhibitors effective in animal models, which were developed without structural knowledge of the protein. Now that we know the shape and size of the drug-binding site within a bacterial model of the protein, this detailed structural information should enable the design of improved drugs which are much more targeted and will ’fit’ much better.”
This new knowledge could have a wider impact on drug design. Dr Alexander Cameron from Imperial College London and the Membrane Protein Laboratory at Diamond explains: “As some drugs are poorly absorbed in the intestine or need to be targeted to the liver, ASBT has also received attention as a pro-drug carrier, capable of transporting various compounds coupled to bile acid. This means that there could be scope to improve a number of drugs tackling different problems, for example, cytostatic compounds targeting liver tumours.”
ASBT is a membrane protein, one of over 7,000 within the human body, of which many are important drug targets. Over 50% of current commercially available drugs target membrane proteins but they are notoriously hard to crystallise – a step that is a pre-requisite in solving protein structures using a synchrotron. Dr David Drew, Royal Society Research Fellow in the Life Sciences Department at Imperial College London said: “Key to the success was to find a suitable detergent that yielded good protein crystals, this arduous task was facilitated greatly by a large-scale stability screen we carried out [1].”
 |
|
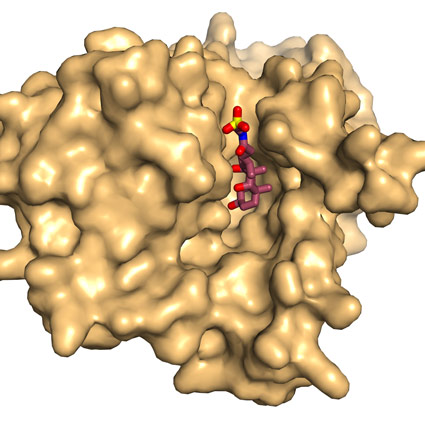
Surface representation of ASBTnm looking from the inside face of the membrane showing bile acid bound within a deep cavity. |
The ESRF and Diamond Light Source were essential to screen their crystals and collect the data used to obtain the structure. Dehydration experiments [2], which improved the resolution of the diffraction data, were also carried out on the crystals.
The research was carried out across four sites: Imperial College London, the Research Complex at Harwell (RCaH), the Membrane Protein Laboratory at Diamond Light Source and the ESRF.
The study was funded by the European Union and the Medical Research Council (MRC), and supported by the Biotechnology and Biological Sciences Research Council (BBSRC), the ERATO IWATA Human Receptor Crystallography Project and the Wellcome Trust.
Principal publication and authors
Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT, N.-J. Hu (a,b), S. Iwata (a,b,c), A.D. Cameron (b,d), D. Drew (a), Nature, 5 October (2011); DOI: 10.1038/nature10450.
(a) Division of Molecular Biosciences, Membrane Protein Crystallography Group, Imperial College, London (UK)
(b) Membrane Protein Laboratory, Diamond Light Source, Harwell Science and Innovation Campus, Didcot, Chilton (UK)
(c) Japan Science and Technology Agency, ERATO, Human Receptor Crystallography Project, Yoshida Konoe, Sakyo-ku, Kyoto (Japan)
References
[1] Benchmarking membrane protein detergent stability for improving throughput of high-resolution X-ray structures, Y. Sonoda et al., Structure. 19, 17-25 (2011).
[2] Improving diffraction by humidity control: a novel device compatible with X-ray beamlines, J. Sanchez-Weatherby et al., Acta Cryst. D65, 1237-1246 (2009).
This news item is published jointly by Diamond Light Source and the ESRF.