- Home
- News
- General News
- The structure of...
The structure of the Kinesin-1 motor-tail complex reveals the mechanism of autoinhibition
09-08-2011
Scientists from Glasgow and Pittsburgh reveal how a kinesin motor is inactivated to save energy in their paper published today in Science. The structural data was collected at the ESRF and the Swiss Light Source.
Share
Kinesins are proteins that transport cargo such as vesicles and mitochondria inside cells, for the normal functioning of cells and neurons. They also play a role in cell division, helping with the physical separation of duplicated chromosomes into the two daughter cells. They are therefore called motor proteins, and they are actually the world’s smallest moving machines, much tinier than any man-made object to date.
Based on their functions in cells, one class of kinesins have been linked to neuronal diseases, such as Alzheimer’s disease. Other classes of kinesins that are involved in cell proliferation have become potential targets for drug development in cancer chemotherapy.
A kinesin motor transports its cargo by moving along the outer surfaces of microtubules which are part of a cell’s cytoskeleton, and are tube-like polymers up to 25 micrometres long. The energy for their movement is provided by the hydrolysis of ATP. But how exactly is a kinesin motor inactivated to avoid the waste of energy when it is not needed for transport in the cell?
This question has now been answered by Kristal Kaan and colleagues from the Beatson Institute for Cancer Research in Glasgow (UK) and the Carnegie Mellon University (USA). The work has been published in the journal Science on 12 August 2011.
Kaan solved the Kinesin-1 motor-tail crystal structure, which led to the identification of a “double lockdown” mechanism of the two motors that make up a functional kinesin. Their work not only shows that the “double lockdown” prevents the kinesin motor from using up ATP when it is not transporting any cargo, it also excludes several other competing hypothesis of how this autoinhibition might happen. It is also important to note that the authors believe the autoinhibition process in Kinesin-1 might be the same for other kinesin motors.
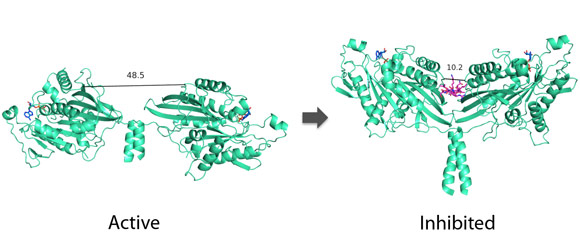 |
|
Kinesin-1 autoinhibition mechanism. (Image credit: H. Kaan, Beatson Institute for Cancer Research). |
References
H. Kaan, D. Hackney and F. Kozielski, The structure of the Kinesin-1 motor-tail complex reveals the mechanism of autoinhibition, Science 333, 883-885 (2011).
Top image: Kinesin-1 autoinhibited.



