- Home
- News
- General News
- Structure of the...
Structure of the membrane domain of respiratory complex I
05-08-2011
This result by a team from MRC Cambridge, published in Nature, will aid in the combat of diseases relating to genetic disorder. Beamlines at the ESRF and the Swiss Light Source provided structural data.
Share
Mitochondria are “cellular power plants”, supplying energy for the organism. They contain their own DNA, about half of which codes for proteins of mitochondrial complex I, the first and largest enzyme in the respiratory “power chain”. Defects in mitochondrial DNA are one of the most common types of human genetic disorder. They occur in about 1 in 5,000 of the population and the majority of these disorders involve complex I. So far there is no treatment for these debilitating diseases, which include neurological impairment, deafness, blindness, muscle weakness and cardiovascular disease.
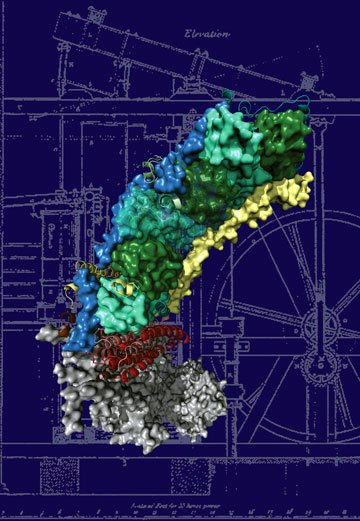
To understand the molecular basis of the disease, as a starting point for drug development, we need to know the structure (molecular architecture) of the protein. Complex I, due to its sheer size, resisted the efforts to determine its structure for a long time. Now a group at the MRC Mitochondrial Biology Unit in Cambridge (UK), led by Dr. Leonid Sazanov, succeeded in the determination of the atomic structure of the membrane-embedded part of the protein, involved in many of the diseases. The structure contains 55 trans-membrane helices and is one of the largest membrane proteins solved to date. This work has been published in the journal Nature on 7 August 2011. The structure shows that the enzyme works by connecting different parts of the protein through mechanical coupling elements, akin to the piston coupling rods in the steam engine. Now we can see for the first time how the most common mutations lead to disorders. The points of these mutations are found in parts of the protein critically important for energy transduction, occurring through coordinated mechanical movements. This knowledge should greatly help the scientists developing drugs against mitochondrial diseases.
References
R.G. Efremov and L.A. Sazanov, Structure of the membrane domain of respiratory complex I, Nature, 476, 414–420 (2011).
Top image: The structure of the membrane domain of respiratory complex I from E. coli. Credits: R. Efremov and L. Sazanov, MRC