- Home
- News
- Spotlight on Science
- Bonding in high-pressure...
Bonding in high-pressure solid CO2 revealed by single-crystal diffraction
22-02-2010
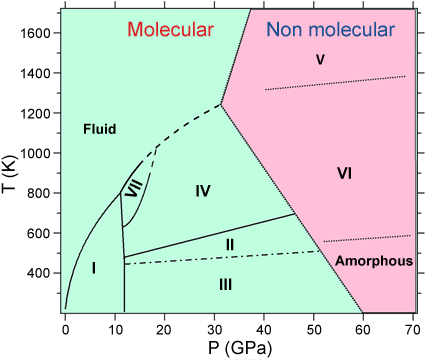
Solid carbon dioxide undergoes a radical change from a molecular solid to a non-molecular “polymeric” state at elevated pressures and temperatures [1]. The polymeric state is characterised by the disappearance of the double bonds between the carbon and oxygen atoms and the formation of new single bonds. Whether this transformation occurs as a result of solid-state chemical reactions between molecules reaching a critical separation, or via intermediate states where molecules gradually distort as pressure increases, remains unknown. To solve this mystery, researchers from Université Pierre et Marie Curie have determined the structure of CO2 in the high-pressure phase IV, the ultimate molecular phase before its polymerisation into phase V.
Share
At ambient conditions, molecular CO2 is linear and composed of two strong covalent double bonds between the carbon and oxygen atoms of length 1.168 Å. By applying pressure at 300 K, carbon dioxide crystallises at 0.6 GPa in the same structure as the one observed at low temperatures, the so-called “dry ice” or phase I. This solid phase is typical of molecular solids, characterised by the large energy separation between the strong intramolecular bonds and the weak intermolecular interactions. Several other crystalline structures have been discovered for CO2 in the last ten years, leading to the the P-T phase diagram presented in Figure 1. In this work we have focused on phase IV, stable above ~11 GPa, which was proposed to be an intermediate state between the low-pressure molecular solid I and the high pressure non-molecular phase V. Indeed, the Pbcn structure proposed from previous experiments [2] suggested that the molecules in phase IV had a bond length 30% larger than in phase I and were no longer linear, exhibiting an OCO angle of 160°. However, such a distortion was in conflict with other experimental and theoretical results, and a strong debate emerged about the actual structure of this phase.
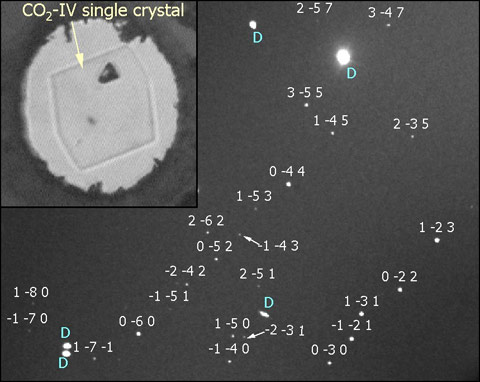
To solve this controversy, we performed single-crystal diffraction experiments on phase IV. Obtaining a single crystal of phase IV is a difficult task as it requires a very fine control of pressure at the high temperature conditions needed to grow phase IV from the melt (T > 800 K). We achieved this by performing single-crystal diffraction using a diamond anvil cell with large opening cones for the incident and outgoing X-ray beam. The cell was designed for high temperatures via resistive heating. We started from a polycrystalline sample of phase IV at 15 GPa and slowly decompressed down to the melting point (11 GPa at 830 K) until a unique crystal seed was stabilised (Figure 2). The crystals were then studied using the hard X-ray sources and angular dispersive setups available at beamlines ID09A and ID27. Because of the small sample size and weak scattering cross-section of CO2, the high photon flux of the ESRF synchrotron and the tiny X-ray spot sizes were crucial to obtaining good diffraction data.
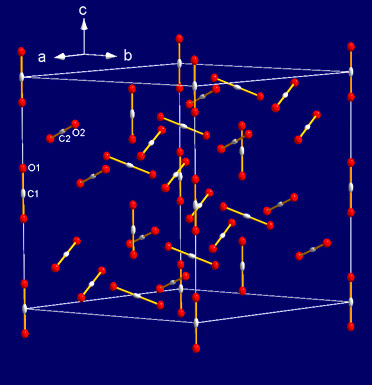
The high quality of the crystal and diffraction data allowed us to solve the structure of phase IV using ab initio methods: it is rhombohedral, space group R-3c, with 8 molecules in the primitive unit cell (Figure 3). The C=O intramolecular bond length was found to be slightly smaller (1.155 Å) than the one of the isolated molecule, in sharp contrast with the intermediate bonding scenario. Moreover, no bending of the molecules was observed. This shows that CO2 remains a purely molecular solid in the pressure range of phase IV.
 |
|
Figure 3. Unit cell of CO2 phase IV (R-3c). Shown is the hexagonal triple unit cell with 24 molecules. |
The stability of this structure was further investigated using theoretical calculations based on density functional theory. The results are in very good agreement with experimental data, both for the structural parameters, and for the Raman and infrared spectra.
These findings also provide evidence of a striking similarity between the high-pressure phase diagrams of CO2 and N2, at least for the molecular phases. Indeed, these two systems crystallise in the same R-3c structure at high pressure, and also have in common the Pa-3 and P42/mnm structures at lower P/T conditions. The R-3c structure is also adopted by the CO molecules at high pressure, showing that it is a favourable compact arrangement for rod-shaped molecules with strong electric quadrupoles. We may thus expect to find the same structure for a number of similar compounds.
In conclusion, our results suggest that polymerisation in carbon dioxide does not occur via intermediate states and is most likely due to solid-state chemical reactions between CO2 molecules. Besides rationalising the phase diagram of molecular CO2, our findings should help in understanding the formation of polymeric phases; indeed, recent studies show that the structure of the polymeric phase formed at high pressure/temperature strongly depends on the one of the starting molecular phase (III, II or IV) [3]. In particular, the structure of the polymeric phase V, which is still being debated, could well be related to that of phase IV. Further experimental and theoretical studies are on their way to determine the behaviour of phase IV when it is compressed in the pressure range of the polymeric states.
References
[1] V. Iota, C.S. Yoo, and H. Cynn, Science 283, 1510 (1999).
[2] J.H. Park, C.S. Yoo, V. Iota, H. Cynn, M.F. Nicol, and T. Le Bihan, Phys. Rev. B 68, 014107 (2003).
[3] J. Sun, D.D. Klug, R. Martoňák, J.A. Montoya, M.-S. Lee, S. Scandolo, and E. Tosatti, Proc. Natl. Acad. Sci. U.S.A. 106, 6077 (2009).
Principal publication and authors
F. Datchi (a), V.M. Giordano (b), P. Munsch (a) and A.M. Saitta (a), Structure of carbon dioxide phase IV: breakdown of the intermediate bonding state scenario, Phys. Rev. Lett. 103, 185701 (2009).
(a) Université Pierre et Marie Curie (Paris 6) & CNRS, IMPMC, Paris (France)
(b) ESRF
Top image: Dry ice - an industrially produced low temperature phase of solid CO2.