- Home
- News
- Spotlight on Science
- The molecular mechanism...
The molecular mechanism of pain relief
08-03-2011
General anaesthetics are one of the most ancient classes of medicine and have reduced human suffering more than any other drug. Despite widespread use, their exact mode of action is not well understood, but a growing body of evidence suggests that they bind specifically to membrane bound receptors in the synapses of nerves. These receptors, called pentameric ligand gated ion channels, send an electrical signal in response to a chemical signal. Two crystal structures of a bacterial homologue of these receptors, in complex with two general anaesthetics, have recently been solved. The results reveal a specific cavity in which the drugs are bound, offer insights into their molecular mechanism, and have the potential to allow the development of improved anaesthetics in the future.
Share
Nerve impulses control processes in the body from movement to sight and thought. They are propagated between nerve cells by chemical signals transmitted across synapses. When an electrical potential arrives at a synapse in response to a stimulus, it must be transmitted across the synaptic cleft – the gap between nerve cells. This is an entirely chemical process, the change in electrical potential triggers the release of neurotransmitters. These molecules then bind to pentameric ligand-gated ion channels (pLIGICs) in the membranes of the adjoining nerve cell. The binding of the molecules stimulates the opening of pLIGICs allowing the rapid influx of cations into the cell, which in turn propagates an electrical impulse along the nerve, thereby transmitting the stimulus. The channels produce local permeability changes in the membranes that are directly correlated to both the concentration of neurotransmitter and its lifetime in the synaptic cleft - changing a self amplifying electrical transmission into a highly-sensitive, graded response. Their receptors are the target of many psychoactive drugs and are probably also the targets of general anaesthetics, but, as they are membrane proteins, structural studies are challenging. Recently, a bacterial homologue of human pLIGICs was discovered and its structure solved [1,2], showing the first molecular details of this class of membrane protein and providing an excellent model for studies of neurotransmission.
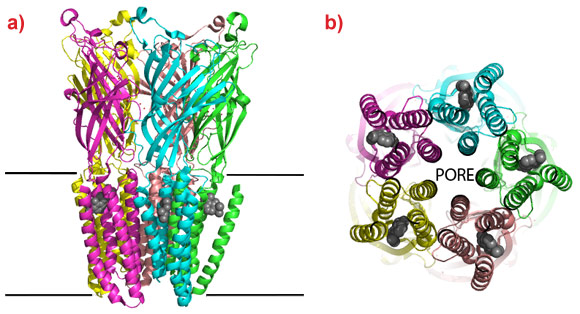
Marc Delarue’s group from the Institut Pasteur and CNRS in Paris (France) have now crystallised a bacterial pLIGIC in complex with two commonly used general anaesthetics, desflurane and propofol. The crystal structures reveal a common binding cavity for the drugs in the membrane bound domain of the ‘cation channel open’ conformation of the protein with interactions between residues that form the cation channel and phospholipids bound to the protein. The cation channel is formed at the interface of the pentamers and the anaesthetics bind in a hydrophobic cleft between four transmembrane helices of each monomer (Figure 1). In order to confirm that this is a physiological relevant binding site, the group conducted electrophysiological tests to show that the anaesthetics alter the ion permeability of the channel and that mutations to the residues that form the pocket identified in the crystal structure also alter the response to the drugs. As the pore is opened and closed by relative movements of the helices to which the anaesthetics are bound, their interaction at this site will affect the channels ability to open and close in response to neurotransmitters. The identification of a binding cavity for two of the most commonly used general anaesthetics opens the possibility to develop a new generation of this important class of analgesics with enhanced characteristics.
The hydrophobic nature of these proteins and their mobility makes obtaining sufficient protein and then subsequently crystallising the system extremely challenging. It also leads to an enormous heterogeneity in crystal quality. The diffraction properties of hundreds of crystals needed to be examined in a synchrotron X-ray beam in order to select those that diffract to a sufficient resolution to allow proper visualisation of drug binding. This type of sample evaluation is only possible at the ESRF Structural Biology beamlines due to their high level of automation of beam alignment and sample handling. The final data sets were collected on beamline ID14-2.
Principal publication and authors
H. Nury (a,b,c,d), C. Van Renterghem (a,b), Y. Weng (e), A. Tran (e), M. Baaden (f), V. Dufresne (a,b), J. Changeux (b,g), J.M. Sonner (e), M. Delarue (c,d) and P. Corringer (a,b), X-ray Structures of general anaesthetics bound to a pentameric ligand-gated ion channel, Nature 469, 428-431 (2011).
(a) Group Récepteurs-Canaux, Institut Pasteur, Paris (France)
(b) CNRS URA 2182, Paris (France)
(c) Unité de Dynamique Structural des Macromolécules, Institut Pasteur, Paris (France)
(d) CNRS URA 2185, Paris (France)
(e) University of California, San Francisco (USA)
(f) CNRS URA 9080, Paris (France)
(g) Collège de France, Paris (France)
References
[1] N. Bocquet et al., Nature 457, 111-114 (2009).
[2] R.J.C. Hilf et al., Nature 457, 115-118 (2009).
Article written by M.W. Bowler, ESRF.
Top image: The molecular mechanism of pain relief (Image credit: M.W. Bowler).