- Home
- News
- Spotlight on Science
- Cardiovascular regulatory...
Cardiovascular regulatory nitric oxide binding to heme proteins investigated using nuclear resonance scattering
26-03-2012
Nitric oxide, NO, plays an essential role as a signal molecule for cardiovascular regulation. A transatlantic cooperation between German, American and ESRF scientists has investigated the binding mechanism of NO to the transporter heme protein nitrophorin, which occurs in the saliva of the blood-sucking Amazon river-based kissing bug Rhodnius prolixus and in the bedbug Cimex lectularius, found worldwide. By using nuclear inelastic scattering (NIS), the researchers were able to pin down heme NO binding modes. They found indications that protonation of the heme carboxyls may modulate NO capture and release. They also noted that the protein matrix influences stability of the heme-NO complex. These results are important for the potential application of this type of NO carrier protein as a cardiovascular drug.
Share
The diatomic molecule nitric oxide, NO, plays an essential role as a signal molecule for cardiovascular regulation, the regulation of cell function and as a neurotransmitter in vertebrates, as well as a toxic defence substance for eliminating invading organisms [1]. NO is toxic in high concentration, its production and transport are strongly regulated. Insects such as the Amazon river-based kissing bug Rhodnius prolixus cause vasodilation by injecting a suite of NO-transporter heme proteins, the nitrophorins, into the tissues and capillaries of their victims [2]. NO dissociates upon dilution and pH rises from ~5−6 in the salivary glands of the insect to ~7.3−7.4 when the saliva is injected into the victim’s tissues.
Nitrophorins are proteins with stable Fe(III)−NO complexes. They represent an ideal system for the study of the dynamic and electronic properties of the heme Fe−NO bond. They are also ideal systems to study the effects of the protein matrix on the properties of heme proteins, since it is still a matter of debate whether the protein matrix induces the very strongly ruffled heme conformation found in nitrophorins and in some other proteins as well, or whether it may be an effect of just a special orientation of the axial histidine to the heme.
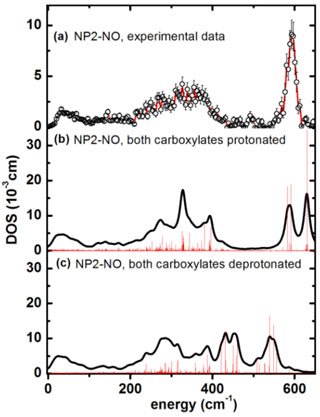 |
Figure 1. Iron vibrational density of states (DOS) for NP2−NO: (a) experimental data, (b) simulation with both heme carboxylates protonated, and (c) with both heme carboxylates deprotonated. |
Nuclear inelastic scattering of synchrotron radiation (NIS) detects molecular vibrations involving iron. It is sensitive to iron-ligand distances, related to the oxidation state of the iron. NIS experiments were carried out at beamline ID18. The NIS data for nitrophorin 2 (NP2) shown in Figure 1a have been calculated by quantum mechanical (QM) density functional theory (DFT) coupled with molecular mechanics (MM) methods (Figure 1b and 1c). The simulations identify iron-NO binding modes (Figure 2), but also functionally relevant low-energy modes (Figure 3). Structure optimisations of the heme and its ligands with DFT retain the characteristic saddling and ruffling only if the protein matrix is taken into account. Thus the heme ruffling in NP2 is a consequence of the interaction with the protein matrix. Furthermore, simulations of the NIS data by the QM/MM calculations suggest that the pH dependence of the binding of NO might be a consequence of the protonation state of the heme carboxyls.
 |
|
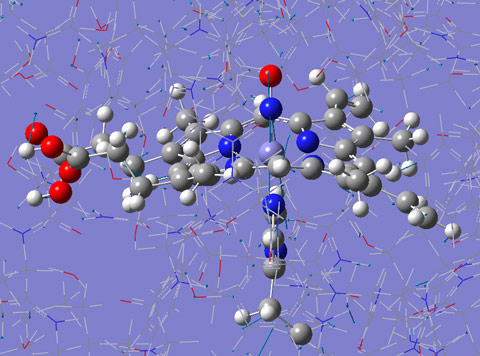
Figure 2. Calculated normal mode of NP2-NO at 630 cm-1. Only the parts of the protein displayed in ball and stick has been treated by quantum mechanical (QM) density functional calculations, the rest of the protein has been treated with the molecular mechanics (MM) approach. This mode dominates the experimentally obtained DOS displayed in Figure 1. Click to see this image as an animation. |
 |
|
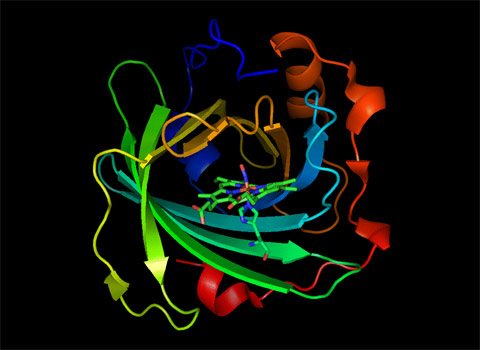
Figure 3. Normal mode of NP2-NO at 70 cm-1 obtained via QM/MM calculations. The displayed mode has substantial doming character which is believed to play a functional role in ligand binding and release. Click to see this image as an animation. |
Principal publication and authors
B. Moeser (a), A. Janoschka (a), J.A. Wolny (a), H. Paulsen (b), I. Fillipov (c), R.E. Berry (c), H. Zhang (c), A.I. Chumakov (d), F.A. Walker (c), V. Schünemann (a), Nuclear Inelastic Scattering and Mössbauer Spectroscopy as Local Probes for Ligand Binding Modes and Electronic Properties in Proteins: Vibrational Behavior of a Ferriheme Center inside a β-Barrel Protein, J. Am. Chem. Soc. 134, 4216 (2012).
(a) Department of Physics, University of Kaiserslautern (Germany)
(b) Institute of Physics, University of Lübeck (Germany)
(c) Department of Chemistry and Biochemistry, University of Arizona, Tucson (U.S.A.)
(d) ESRF
References
[1] S. Moncada et al., Pharmacol. Rev. 43, 109 (1991).
[2] J.M.C. Ribeiro et al., Science 260, 539 (1993).
Top image: Nuclear inelastic scattering captures iron movement in heme proteins.



