- Home
- News
- Spotlight on Science
- Phase contrast imaging...
Phase contrast imaging of lipid membranes in solution
13-04-2012
A lipid membrane in solution at its native thickness of less than 5 nm has been imaged for the first time using coherent X-rays. Researchers witnessed the formation of a bilayer from two lipid monolayers. Lipid bilayers are important in nature as they are the basis for cell membranes.
Share
Biomolecules form large-scale supra-molecular aggregates by self-assembly, forming functional units made of non-covalent bonds. The properties of biomolecular assemblies depend on the molecular to supra-molecular structure. An excellent example of supra-molecular assembly is the formation of lipid bilayers which represent the basic building blocks for biological membranes, nature's most important interface.
As in many complex fluids and biomolecular assemblies, functionally relevant states and properties depend intricately on hydrated environmental conditions and the parameters of the aqueous solvent, for example pH, ionic strength and molecular concentrations. X-ray and neutron diffraction techniques are compatible with these environmental conditions, and have therefore become invaluable tools to study so-called soft matter or complex fluid samples. This is in sharp contrast to electron probing beams, which would enable similar resolution, but necessitate vacuum conditions.
A major problem with conventional diffraction, however, is that it yields only the averaged structure of a large ensemble of identical structures. Furthermore, substantial modelling is often required. In other words, the diffraction data cannot usually be inverted to produce an image. If the sample is structured on a hierarchy of length scales, unravelling its structure by diffraction is generally not possible. Small-angle X-ray diffraction from membrane suspensions, for example, requires several microlitres of relatively homogeneous samples. To turn diffraction into true imaging, intensive research over the last 15 years has been devoted to solving the so-called phase problem. In fact, imaging has been achieved when the diffraction experiment is carried out with sufficiently coherent X-ray radiation. The irony of this triumph is that, in order to concentrate coherent light onto the sample, it was almost always necessary to work with dry or fixated samples, primarily for dose issues and radiation damage. In this way, one of the strongest advantages of the X-ray probe was lost, and the native and functional structure of most complex fluids samples was again inaccessible.
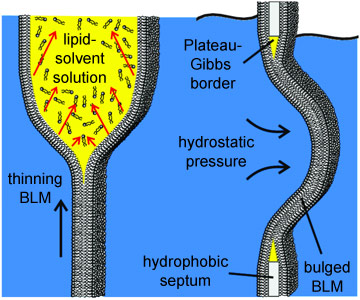
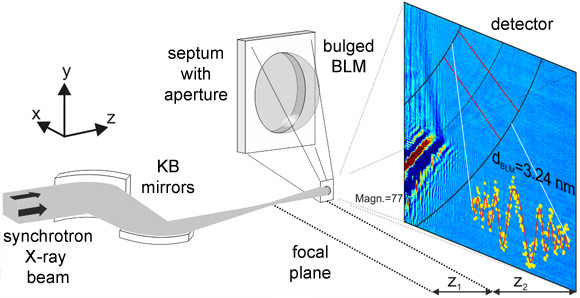
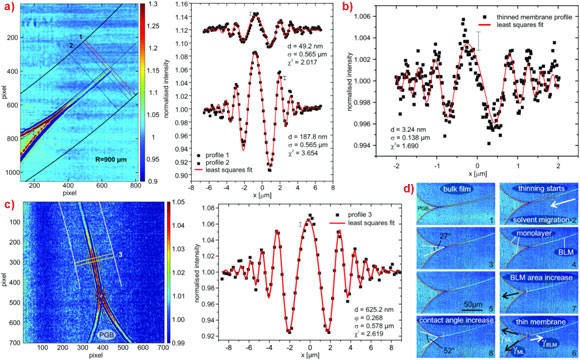
Researchers from the Georg-August-Universität Göttingen and the Max-Planck Institute for Dynamics and Self-Organization, in collaboration with colleagues from the ESRF, now report the first X-ray imaging of a hydrated lipid membrane in solution at its native thickness of less than 5 nm corresponding to two molecular layers, the so-called lipid bilayer. This became possible by using a particularly dose efficient approach based on free space X-ray propagation, and an analysis strategy which is a combination of near-field diffraction and imaging. The researchers were able to resolve thickness and density of the membrane along the direction perpendicular to the interface, as well as imaging its contour. For the experimental demonstration, they worked in two steps. First, a single, freely-suspended lipid bilayer was studied using a well-known setup of membrane electrophysiology, the so-called black lipid membranes (BLM), an established model system in membrane biophysics. Figures 1 and 2 present the formation of a BLM and the experimental setup. The bilayer was spanned between two separated, aqueous compartments. This allows studies of functional transport across the bilayer at controlled compositional and environmental parameters. The membrane was bulged by hydrostatic pressure, and placed a few millimetres behind the focal plane of a highly efficient and partially coherent elliptical mirror system of the nano-imaging station ID22NI. This quasi-spherical illumination leads to a magnified hologram of the sample which is then recorded a few metres behind the sample by a fast read-out low-noise detector system (FReLoN). The Fresnel oscillations in this hologram corresponding to the native lipid bilayers with a thickness in the range of d = 5 nm were recorded and analysed with respect to the local structure at molecular length scales (see Figure 3a,b).
In the second part of the experiment, dynamic interface processes were addressed, namely the formation of a bilayer from two monolayers (see Figure 3c,d). To this end, the macroscopic sample chamber was replaced by a microfluidic device designed to observe the formation of a reconstituted lipid bilayer by fusion of two surfactant monolayers using a controlled flow of oil and water in microfluidic channels and mixers. In both systems, the membrane can be electrically charged by the inclusion of electrodes within the aqueous channels.
Straightforward extensions of this approach should enable the extraction of the local interface density profiles of membranes and membrane based materials. More generally, it will allow structure analysis of soft matter interfaces at near-molecular resolution under hydrated conditions, closing the gap between conventional scattering studies, carried out over large ensembles, and conventional microscopy studies. Combining advantages of resolution, interaction volume and complexity of the system studied, the experiment paves the way for the controlled investigation of complex biochemical and physical phenomena, particularly soft interfacial phenomena. The combination of the method with microfluidics can yield structural information on the interfaces during (hydro)dynamic processes, such as the formation of bilayers, thinning or bulging, as well as membrane fusion, and all these on a length scale approaching only a few nanometres.
Further information
A movie of a bulging membrane showing the 'Marangoni effect' in the film
Principal publication and authors
A. Beerlink (a,b), S. Thutupalli (c), M. Mell (a,d), M. Bartels (a), P. Cloetens (e), S. Herminghaus (c), and T. Salditt (a), X-ray propagation imaging of a lipid bilayer in solution, Soft Matter 8, 4595-4601 (2012).
(a) Institut für Röntgenphysik, Georg-August-Universität Göttingen (Germany)
(b) current address: Deutsches Elektronen-Synchrotron, PETRA III, Hamburg (Germany)
(c) Max-Planck-Institute for Dynamics and Self-Organization, Göttingen (Germany)
(d) current address: Mechanics of Biological Membranes and Biorheology, Departamento de Quimica Fisica I, Universidad Complutense, Madrid (Spain)
(e) ESRF
Top image: Phase contrast imaging of a lipid membrane.