- Home
- News
- Spotlight on Science
- Nanofilm molecular...
Nanofilm molecular structures at the mica-water interface
30-05-2012
The interfacial structure of a number of surfactant and polymer/surfactant systems has been unravelled through synchrotron X-ray reflectometry measurements at the solid-liquid interface. Understanding the intricate structural details of soft matter nanofilms is important for a wide range of industrial and biomedical applications and fundamental to our knowledge of many biological and natural processes.
Share
Amphiphilic molecules such as surfactants and surface-active polymers can readily adsorb at the solid-aqueous interface and form various organised structures. Understanding the characteristics and properties of such adsorbed molecular structures at the solid surface is important to many chemical and industrial applications and natural processes including personal care products, detergency and biolubrication.
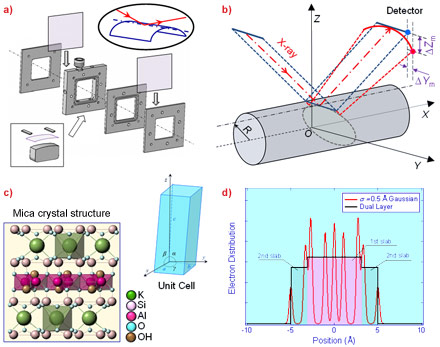
Molecularly smooth muscovite mica, although widely used as a model substrate in surface-sensitive techniques such as atomic force microscopy (AFM), electron microscopy and surface force apparatus, up to now has not been considered as a suitable substrate for X-ray reflectometry (XRR) studies on nanofilms due to the difficulties in obtaining flatness over a sufficiently large area of mica. We have overcome this difficulty using a liquid cell (Figure 1a) that implements a simple “bending mica” method [1], where the enhanced rigidity of the mica sheet along its bending axis would facilitate sufficient flatness to perform XRR measurements along the axis. Experimentally, a detailed consideration of the bending curvature on the reflectivity has guided the setup to account for the divergence of the reflected beam (Figure 1b). In addition, the effect of the crystal truncation rods due to mica’s layered crystal structure (Figure 1c) on the overall reflectivity must be accounted for in the data analysis. This challenge is met by modelling mica’s electron density distribution as a dual layer (Figure 1d).
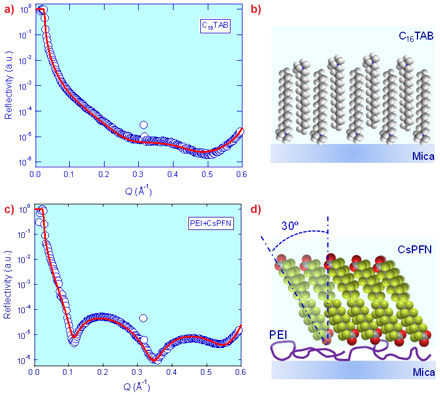
This method has been successfully employed on BM28 (XMaS) and ID10B to reveal detailed structural information of a number of soft nanofilms as exemplified in Figure 2. Hexadecyltrimethylammonium bromide (C16TAB), a commonly used cationic surfactant, is found to form an interdigitated bilayer 32.0 Å in thickness with a full surface coverage at the highly charged mica-water interface (Figure 2a,b) at its critical micelle concentration (cmc). This result contrasts with previous AFM imaging data which showed the formation of cylindrically shaped surface aggregates. It implies that the localised force field or nanoconfinement might have induced the morphology of surface aggregates as observed with AFM, which is different from the intrinsic aggregation structure formed under quiescent conditions in XRR. This has general implications for our fundamental understanding of surfactant adsorption mechanisms at the solid-liquid interface, and in turn, how surfactant layers may tailor surface chemistry and mediate inter-surface interactions. As another example, the interaction between poly(ethyleneimine) (PEI), a positive charged polymer, and cesium perfluorononanoate (CsPFN), a fluorinated anionic surfactant at its critical micelle concentration, is shown to lead to the formation of a CsPFN bilayer 18.0 Å in thickness atop the PEI layer. The CsPFN fluorocarbon surfactant tails are found to tilt at 30.6° with respect to normal, with a full surface coverage. Such detailed structural information will be beneficial to optimising the synergetic effect of co-adsorption of surfactant-polymer complexes and achieving desired surface structures for applications such as detergency, paints, cosmetics and lubrication.
The liquid cell used here can be applied to study the structure of a wide range of systems at the mica-water interface. These include the interactions between polyelectrolytes and lipids, structuring of ionic liquids, conformations of polymer brushes and lipid liposomes. The results will provide molecular details of these soft nanofilms at the highly charged mica-water interface previously unattainable with optical techniques such as ellipsometry, optical reflectometry and Fourier transform infrared spectroscopy. Such information will further improve our fundamental understanding of soft matter adsorption at the solid-liquid interface, relevant to a number of industrial applications and biological processes.
Principal publication and authors
Synchrotron XRR study of soft nanofilms at the mica-water interface, W.H. Briscoe (a), F. Speranza (a), P. Li (b), O. Konovalov (c), L. Bouchenoire (d,e), J. van Stam (f), J. Klein (g), R.M.J. Jacobs (h), and R.K. Thomas (b), Soft Matter 8, 5055-5068 (2012).
(a) School of Chemistry, University of Bristol (U.K.)
(b) Physical and Theoretical Chemistry Laboratory, University of Oxford (U.K.)
(c) ESRF
(d) XMaS, the UK-CRG, ESRF, Grenoble (France)
(e) Department of Physics, Oliver Lodge Laboratory, University of Liverpool (U.K.)
(f) Department of Chemistry and Biomedical Sciences, Karlstad University (Sweden)
(g) Department of Materials and Interfaces, Weizmann Institute of Science, Rehovot (Israel)
(h) Department of Chemistry, Chemistry Research Laboratory, University of Oxford (U.K.)
References
[1] W.H. Briscoe et al., Journal of Colloid and Interface Science 306, 459-463 (2007).
Top image: X-ray reflectometry using a “bending mica” method yields interfacial structural details of soft nanofilms in aqueous media.