- Home
- News
- Spotlight on Science
- Ionic spatial distribution...
Ionic spatial distribution at polarised mercury surfaces probed by X-ray reflectivity
22-08-2012
The interface between mercury and an electrolyte solution has been used for more than 50 years as a model system to test the applicability of theories for the electric double layer in order to quantitatively interpret ion-dependent macroscopic surface tension data for such systems. Using X-ray reflectivity, scientists have obtained an angstrom-resolved picture of the spatial distribution of ions in the vicinity of a polarised Hg surface exposed to various molar electrolyte solutions. This study highlights several peculiar features of the Hg/electrolyte interface that have been omitted by theoretical models both past and present. It also provides the first molecular view of ionic distributions in the vicinity of a charged interface since the pioneering work by Graham in 1947 on electric double layers in colloidal systems.
Share
Ions have been classified according to the “Hofmeister series” for more than 120 years [1]. According to this series, ions with the same charge exhibit different effects depending on their nature. These ion specific effects have been shown to play a central role in numerous processes such as protein stability, amyloidosis, enzymatic activity, DNA structure and tropospheric ozone destruction. The relative contributions of ion size, ion polarisability, or ion hydration to such ion-specific effects remains the object of numerous debates that are difficult to settle because robust experimental data on ion concentration profiles at model charged surfaces are lacking.
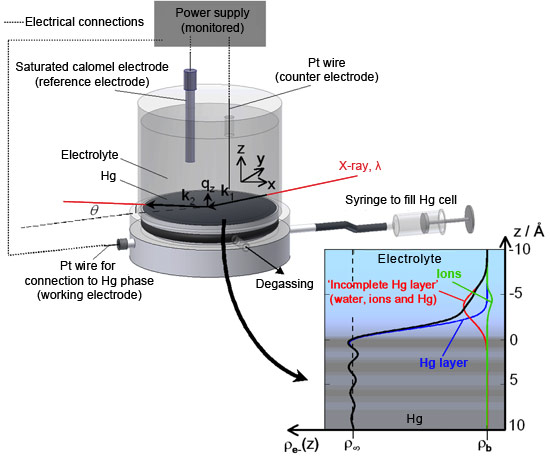
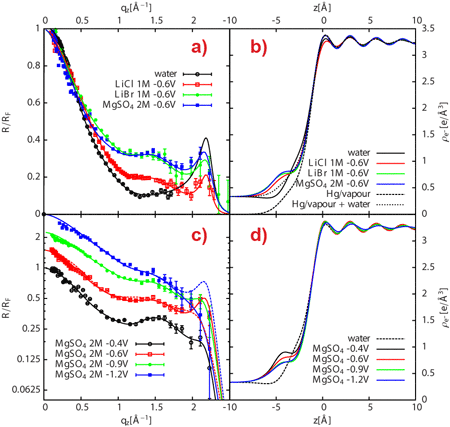
Now, a team lead by scientists from Nancy University and CEA/CNRS-Saclay, in collaboration with the staff at beamlines ID10 and ID15, have succeeded in measuring the ionic distributions at buried and polarised Hg-electrolyte solution interfaces with unprecedented accuracy. High-energy X-rays were used to detect the changes in reflectivity resulting from the presence of various ionic species at the mercury surface, with the mercury surface acting as a mirror for X-rays. Detailed analysis of the reflectivity data yielded information about the ionic profiles close to the interface with sub-angstrom spatial resolution (Figure 1).
The Hg/electrolyte interface has long been known to exhibit ion specific effects as its surface tension depends on both applied potential and ion nature, a feature classically treated on the basis of the Lippmann-Graham macroscopic thermodynamic description. By reconstructing the reflectivity profiles obtained from the Hg surface subjected to various applied potentials and exposed to molar concentrations of LiBr, LiCl and MgSO4 electrolytes, it was possible to show that both the degree of polarisation of Hg and the anion nature significantly impact the liquid structure of the Hg close to the interface (Figure 2). In the case of the Hg/MgSO4 electrolyte interface, the interfacial roughness obtained from X-ray reflectivity data was shown to concur with predictions from capillary wave theory. Conversely, the X-ray reflectivity-determined ion surface excess concentrations were found to be significantly larger than those yielded by macroscopic potential-dependent surface tension data (electrocapillary curve) for the same interface. The observed discrepancy can be assigned to the complex layered structure of the liquid Hg at the interface and to the presence of an incomplete Hg layer composed of water, ions and Hg atoms that gradually protrude from the mean Hg surface location, thus forming a diffuse liquid interphase. These results suggest that even the sign – not only the numerical value – of the ion surface excess determined from surface tension measurements might be incorrect. These findings have strong implications for the understanding of the composition and structure of metal/electrolyte interfaces, and clearly call for a re-examination of past theoretical modelling. Future models should in particular take into account all the fine details of the Hg surface in order to appropriately decipher ion-specific processes. In particular, potential- and ion-dependent electronic properties of the polarised Hg surface (incomplete Hg topmost layer and Hg layering) are important factors that have been omitted both in the traditional derivation of double layer ion composition from electrocapillary data and in their recent interpretation employing the anisotropic hypernetted chain (AHNC) theory [2].
Principal publication and authors
J.F.L. Duval (a), S. Bera (b,c), L.J. Michot (a), J. Daillant (b,d), L. Belloni (b), O. Konovalov (e), D. Pontoni (e), Physical Review Letters 108, 206102 (2012).
(a) Laboratoire Environnement et Minéralurgie, Nancy-Université, CNRS UMR 7569, Vandoeuvre-lès-Nancy (France)
(b) Laboratoire Interdisciplinaire sur l'Organisation Nanométrique et Supramoléculaire, UMR 3299 CEA/CNRS, Saclay (France)
(c) Department of Physics, Northern Illinois University, Dekalb (USA)
(d) Synchrotron Soleil, Saint-Aubin (France)
(e) ESRF
References
[1] F. Hofmeister, Arch. Exp. Pathol. Pharmakol. 24, 247 (1888).
[2] E. Wernersson, R. Kjellander, and J. Lyklema, J. Phys. Chem. C 114, 1849 (2010).
Top image: The X-ray reflecting mercury surface is visible near the bottom of the transparent cylindrical sample cell containing the electrolyte solution. Here the cell is mounted in the high-energy microdiffraction instrument of beamline ID15A.