- Home
- News
- Spotlight on Science
- Hydrated minerals...
Hydrated minerals from the early solar system probed by Fe-XANES spectroscopy
26-09-2012
Meteors occasionally survive impact with the Earth's atmosphere and fall down to Earth as meteorites. These extra-terrestrial rocks let us sample the composition of distant bodies in the Solar System including asteroids, Mars and the Moon. In this study, X-ray spectroscopy, Fe-XANES, was used to determine the crystal chemistry of iron in carbonaceous chondrite meteorites, giving a unique insight into an aqueous alteration process that occurred on their parent asteroids or planets.
Share
Each year, a few meteorites crash down onto the Earth’s surface. These extra-terrestrial rocks show a variety of mineralogy and provide us with an opportunity to sample other Solar System bodies. The carbonaceous chondrite meteorites are the most rare and probably the oldest and most primitive pieces of the Solar System ever found on Earth. They are “fossils” from the time the Solar System was created 4.568 billion years ago and their composition is believed similar to that of the sun. They are made up of mineral phases, together with some amount of carbonaceous materials. Although primitive in terms of chemistry, these meteorites provide evidence of geological processes that existed on their parent asteroid or planet. Indeed, their mineralogy appears to have been modified by reactions with an aqueous fluid, providing the oldest evidence of liquid water in the Solar System. Carbonaceous chondrites could even be the origin of terrestrial amino acids as well as a possible source of terrestrial water [1]. The mineralogy of these samples is dominated by phyllosilicates, which are “water” bearing minerals. Some samples can contain up to 20 wt% equivalent H2O that would have been incorporated during an aqueous alteration process that took place on their parent asteroid or planet.
The parent bodies of most meteorites are found in the Solar System’s main asteroid belt situated between Mars and Jupiter. The carbonaceous chondrites have been related to a specific family of asteroids, the C-types, by spectroscopic similarities. Both have a low albedo (a few percent only) and show absorption features in the vis-NIR, attributed to the presence of oxidised Fe3+ bearing minerals.
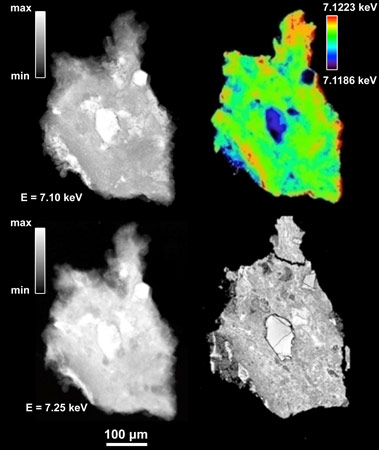
In order to determine the crystallographic nature of the Fe-bearing phases in carbonaceous chondrites, Fe-XANES spectroscopy was performed at ID21, the ESRF’s X-ray microscopy beamline. As these objects are heterogeneous at the micrometre scale, we made use of ID21’s full-field setup [2]. A series of eight carbonaceous chondrites was measured, seven samples from the CM meteorite classification group, as well as the meteorite Orgueil (from the CI group). We selected samples from these two families because alteration minerals had been described previously, and because of the reported freshness of the samples, i.e. limited if no alteration during the terrestrial residence of the samples. Among the samples, five are meteorites that were seen falling and were subsequently recovered (so-called falls). The other three samples were found during meteorite search campaigns in Antarctica funded by NASA. Figure 1 shows X-ray transmission images of one of the samples, the grain of Murchison (CM), and its SEM image for comparison.
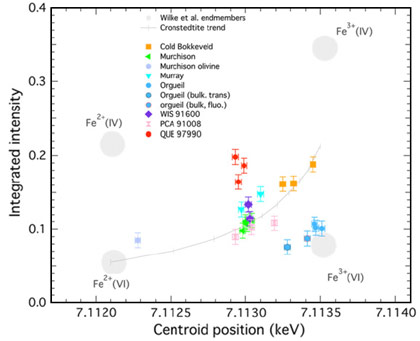
Quantitative information on the Fe containing minerals in the samples was obtained by analysis of the data. These measurements were used to deduce the average redox state of iron (the Fe3+/Fe2+ ratio) in the samples. A range of oxidation states was found. This permitted us to identify a relationship between oxidation state and the evidence for aqueous alteration derived by petrographic observation. There is an increase in the Fe3+/Fe2+ ratio, from moderately altered samples (like the CM Murchison) to samples that were heavily altered by an aqueous fluid (like the CM Cold Bokkevelt). At one extreme lies the meteorite Orgueil (the reference sample for Solar System composition), in which all iron occurs as Fe3+. Figure 2 shows a plot of the XANES peak intensity and position for the measured samples and compares them with known values for materials with various iron oxidation states. Thus we have developed a method that enables us to make the first quantification of the aqueous alteration in primitive meteorites. This is essential for understanding the physico-chemistry of asteroidal waters (pH, water-rock ratio, D/H ratio, etc.).
We also unambiguously identified cronstedtite, a phyllosilicate from the serpentine mineral family. This phase, which is quite rare on Earth, is a phyllosilicate containing both tetrahedrally- and octahedrally- coordinated Fe3+. Most likely, meteorite cronstedtite was formed by the hydrolysis of a primary Fe-metal/silicate mixture.
Our results explain the optical spectra of carbonaceous chondrites. For example, the presence of absorption at 0.7–0.8 and 0.9-1.0 µm in the reflectance spectra of CM can be related to the presence of phyllosilicates containing both Fe2+ and Fe3+ (Fe2+-Fe3+ charge transfer band). Whereas iron species in Orgueil are predominantly Fe3+, which explains the lack of absorption at 0.7 micrometres in their vis-NIR reflectance spectra.
Finally, our study confirms that the Orgueil meteorite has a mineralogy clearly distinct from other carbonaceous chondrites. By reconstructing the trajectory of Orgueil when it fell (in 1864 in southwestern France), it was shown that a cometary origin was more likely than an asteroidal one. Today, the mineralogy of comets is difficult to determine but solid experimental information is expected by 2014 when the European lander PHILAE (ESA/ROSETTA) will touch down on the comet 67P/Churyumov-Gerasymenko.
Principal publication and authors
The redox state of iron in the matrix of CI, CM and metamorphosed CM chondrites from Fe-XANES spectroscopy, P. Beck (a), V. De Andrade (b), F.-R. Orthous-Daunay (a), G. Veronesi (c), M. Cotte (c), E. Quirico (a), B. Schmitt (a), Geochimica et Cosmochimica Acta 99, 305–316 (2012).
(a) UJF-Grenoble 1 / CNRS-INSU, Institut de Planétologie et d’Astrophysique de Grenoble (IPAG), Grenoble (France)
(b) NSLS II, Brookhaven National Laboratory, Upton (USA)
(c) ESRF
References
[1] F. Albarede, Nature 461, 1227-1233 (2009).
[2] V. De Andrade, J. Susini, M. Salome, O. Beraldin, C. Rigault, T. Heymes, E. Lewin, O. Vidal, Anal. Chem. 83, 4220–4227 (2011).
[3] M. Wilke, F. Farges, P.E. Petit, G.E. Brown and F. Martin, Am. Mineral. 86, 714–730 (2001).
Top image: Full-field X-ray transmission image of a fragment of the Murchison meteorite (CM), at an energy of 7.10 keV.