- Home
- News
- Spotlight on Science
- The dark side of...
The dark side of KillerRed
19-12-2012
KillerRed, a red fluorescent protein, generates highly-cytotoxic reactive oxygen species when illuminated with strong light. The molecular mechanism of the laser-induced photo-conversion that results in a photobleached state of the molecule has been elucidated for a highly photosensitive mutant of KillerRed by using X-ray crystallography combined with UV/Visible spectroscopy. These results will help in the design of new improved photosensitive or photoresistant fluorescent proteins for chomophore-assisted light inactivation and may even provide the basis for enhanced photodynamic cancer therapy.
Share
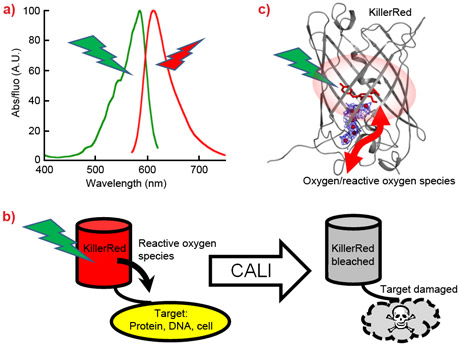
Over-illuminated fluorescent proteins undergo significant transformations that irreversibly result in photobleaching and thus loss of their fluorescence. This phenomenon is a nuisance for common fluorescence-based imaging applications, while it is turned into an advantage for a few specific techniques, which include in situ photo-activation of photosensitising fluorescent proteins. Photosensitising fluorescent proteins contain chromophores that generate reactive oxygen species upon illumination. They are used for the selective inactivation of biological molecules in chomophore-assisted light inactivation (CALI), or for light induced destruction of targeted cells, which has potential as photodynamic therapy for cancer treatment. KillerRed is the first engineered genetically-encoded photosensitiser. It has an exploitable photo-induced cytotoxicity 1000-fold more than other fluorescent proteins. In situ generation of cytotoxic species using genetically-encoded biological molecules avoids problems concerning the introduction of exogenous chemical agents into living organisms [1]. KillerRed’s production of toxic reactive oxygen species is induced by prolonged excitation in the absorption band of the chromophore (Figure 1a), and it is concomitantly associated with the photobleaching of this molecule (Figure 1b) [1]. Understanding the mechanisms that lead to photobleaching is crucial in order to engineer further optimised fluorescent proteins. However, this complex process has so far remained poorly characterised.
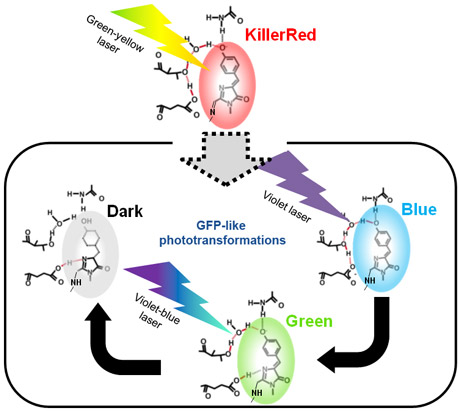
Using facilities at the ESRF cryobench laboratory and the on-line microspectrophotometer, we have recently combined X-ray crystallography with UV/Visible spectroscopy to study a green emitting mutant of KillerRed that is remarkably sensitive to bleaching. The three-dimensional structure of this mutant consists of a β-barrel housing a chromophore typical of the green fluorescent protein (GFP) family. Its crystal structure exhibits an internal channel that is long and water-filled (Figure 1c), similar to that of the wild type KillerRed. This distinctive feature has been proposed to account for the cytotoxic properties of KillerRed [2,3]. Indeed, molecular dynamics simulations have indicated that this channel facilitates the traffic of oxygen species in or out of the protein and provides access to the chromophore [4]. Interestingly, in the process of reactive oxygen species production, the red chromophore of KillerRed is photo-transformed into a “prebleached” GFP-like form (blue/green) prior to reaching its ultimate photobleached dark state. In crystallo spectroscopy has allowed us to investigate the mechanism of the laser-induced photo-transformations leading to photobleaching (Figure 2). The loss of fluorescence can be attributed to irreversible structural modifications such as the disruption of the aromaticity of the chromophore.
Comparison of the KillerRed mutant structure with other less photosensitive fluorescent proteins reveals that its photo-sensitivity is related to the flexible nature of the chromophore environment. This structural flexibility probably facilitates light-induced chromophore movement and reduces the possibility of the chromophore returning to its initial position. Chromophore destabilisation even appears to increase the porosity of KillerRed and, consequently, the reactive oxygen species diffusion process. These findings are of great interest for the structure-guided design of new photoresistant fluorescent proteins adapted to fluorescence-base imaging applications and optimised photosensitisers for the photodynamic therapy of cancer.
Principal publication and authors
GFP-Like Phototransformation mechanisms in the cytotoxic fluorescent protein KillerRed unraveled by structural and spectroscopic investigations, E. de Rosny (a), P. Carpentier (b), J. Am. Chem. Soc. 134, 18015 (2012).
(a) Institut de Biologie Structurale, UJF, CEA, CNRS (Grenoble)
(b) ESRF
References
[1] M.E. Bulina et. al., Nat Biotechnol. 24, 95 (2006).
[2] P. Carpentier et. al., FEBS Lett. 583, 2839 (2009).
[3] S. Pletnev, et. al., J Biol Chem. 284, 32028 (2009).
[4] A. Roy, et. al., Photochem Photobiol Sci. 28, 1342 (2010).
[5] M4D Cell-imaging platform, IBS
Top image: Light-induced killing of HeLa cells using KillerRed, visualised by fluorescence microscopy, before illumination on the left, after on the right (courtesy of J-P. Kleman, M4D Cell-imaging platform, IBS [5]).