- Home
- News
- Spotlight on Science
- Losing crystallinity...
Losing crystallinity under pressure: structural description of the pressure-induced amorphisation in zirconium tungstate
04-10-2007
The crystalline structure of zirconium tungstate (ZrW2O8) is highly flexible and this gives rise to its unusual behaviour including negative thermal expansion, where the crystalline lattice contracts even though the bonds lengthen with increasing temperature, and pressure-induced amorphisation, where there is apparent loss of all long-range order in the material on increasing pressure; the origin of this latter effect remained unclear. Researchers from the UK have succeeded in modelling and refining the structure of the amorphous material, produced at pressures exceeding 4 GPa, by combining in situ and ex situ neutron and X-ray total scattering measurements from the PEARL/HiPr and GEM instruments at ISIS and ESRF beamlines ID27 and ID31. Investigation into the formation of the amorphous material has also lead to a mechanism for the pressure-induced amorphisation process in ZrW2O8, which may be generally applicable to other framework structures based on silica, which also display negative thermal expansion and amorphisation phenomena.
Share
The pair distribution function lends itself particularly well to the investigation of disordered and nanometric materials as we can obtain information over interatomic, domain and longer real-space length scales simultaneously; other techniques are sensitive to local environments or long-range structure only. This method in its most general sense involves the collection of total scattering data composed of the sharp Bragg (ordered) contribution and the broad diffuse (disordered) component of neutron and X-ray diffraction patterns. These data are naturally highly complementary due to the contrasting scattering powers of atoms in X-ray and neutron beams. Combining these datasets allows refinement of disordered structural models and can lead to a mechanism for the amorphisation process when compared to atomistic simulations.
The behaviour of ZrW2O8 under extreme conditions is of immense technological and scientific interest. This is largely due to its flexible crystalline framework—built from corner linked ZrO6 and WO4 polyhedral units—that gives rise to rare phenomena such as its well-known negative thermal expansion (NTE) whereby its volume reduces as temperature increases [1]. This network structure may also enable pressure-induced amorphisation (PIA), seen previously in X-ray diffraction and Raman studies [2], where the crystalline structure disorders on angstrom length-scales upon compression. PIA in ZrW2O8 starts at 1.5 GPa and is complete by 3.5 GPa. The mechanism for PIA is unknown, though coordination changes [3], the involvement of the formation of the α-U3O8-type phase [4], freezing of random polyhedral orientations [2] and kinetically inhibited decomposition towards a mixture of ZrO2 + WO3 [5]. Theoretical studies suggest there is a link between PIA and NTE [6] and that both are related to the inherent flexibility of the arrangement of the polyhedral units.
The first step in understanding the mechanism of PIA is to study the structure of the PIA material itself. This poses particular problems for classical diffraction techniques and requires new methods of study [7] as the PIA material is characterised by the complete loss of Bragg diffraction and standard crystallography does not apply.
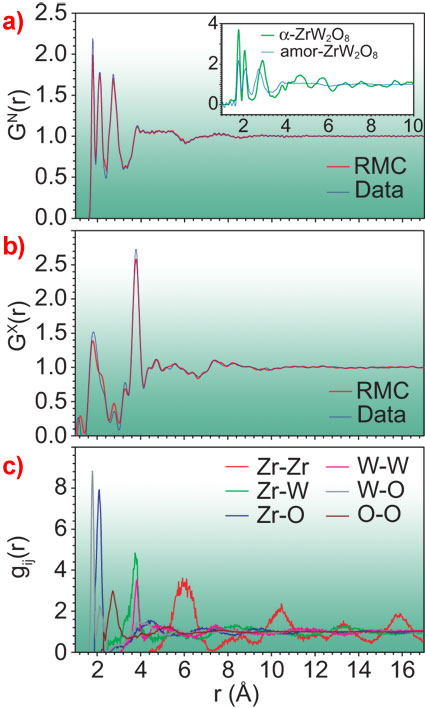
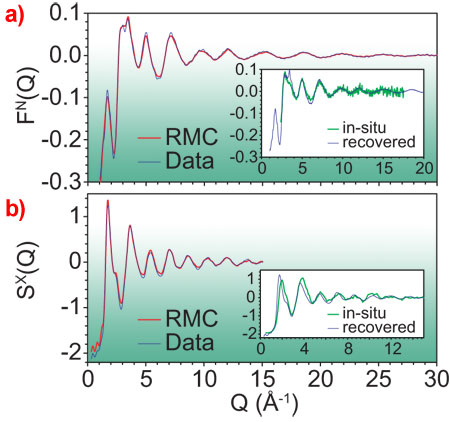
The combination of neutron (from the PEARL/HiPr and GEM instruments at ISIS) and X-ray datasets (taken at ESRF beamlines ID27 and ID31) was invaluable for this study as X-ray and neutron diffraction is dominated by contributions involving tungsten and oxygen atoms, respectively. Thus, by comparing individual peaks in the contrasting datasets (Figures 1a and 1b) the authors can identify the contribution of each Zr-O, W-O, Zr-W, etc. bond (the gij(r), Figure 1c) to the total signals. Furthermore, an atomistic model can be refined using the Reverse Monte Carlo (RMC) code RMCProfile [7]. The modelling process involved a disordering of an initial model (here based on the low-pressure α-phase crystal structure) through the formation of additional linkages and subsequent relaxation, and a refinement to the X-ray and neutron structure factors (Figure 2) to yield a robust solution (Figure 1).
Inspection of the neutron and X-ray distribution functions GN(r) and GX(r) [8], Figures 1a and 1b, immediately shows the true amorphous nature of the PIA material. There are no significant oscillations that would be indicative of regularity in a crystalline structure at length-scales longer than about 4 Å (next-next neighbour distances), though there is still some inherent periodicity in the Zr positions, even to long range (the red function in Figure 1c). This is intriguing and indicates that PIA materials can in fact retain some periodicity which may not be evident from diffraction studies, particularly when the periodic correlations have a low contribution to the scattering data.
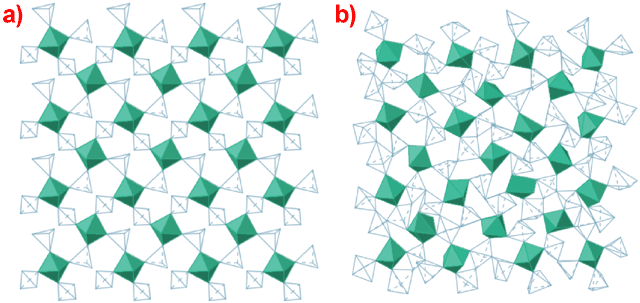
Figure 3 shows the difference between the α-ZrW2O8 crystalline phase [9] and the PIA material, obtained via reverse Monte Carlo (RMC) techniques. Several points are evident: again the semi-periodic distribution of the Zr octahedra (though their relative orientations are disordered); no bond breaking is involved; changes in overall coordination are small (Zr retains 6-fold and W is now 5-fold); there are additional linkages, via the formation of new W-O-W bonds. We can quite easily see the relationship between the α-ZrW2O8 structure and the PIA material and the transition mechanism becomes clear. The mechanism is related to the NTE process; there are many modes associated with NTE and all will soften with increasing pressure. Of these, some will soften completely at various wave vectors producing an amorphous structure via a displacive phase transition (the density of the PIA material is 26% higher than that of the α-ZrW2O8 form at ambient conditions). The magnitude of the displacements associated with these condensed modes is sufficient to bring atoms close enough together to allow the formation of new bonds. Similar mechanisms may also play a role in the PIA of zeolites, some of which also display NTE.
In conlusion, data gathered from both neuton and X-ray studies has led to the identification of a mechanism for pressure-induced amorphisation in ZrW2O8 and yields an amorphous structure.
References
[1] J.S.O. Evans, W.I.F. David, and A.W. Sleight, Acta Crystallogr. Sect. B 55, 333 (1999).
[2] C.A. Perottoni and J.A.H. da Jornada, Science 280, 886 (1998).
[3] T. Varga et al., Phys. Rev. B 72, 024117 (2005).
[4] A. Grzechnik et al., Chem. Mater. 13, 4255 (2001).
[5] A.K. Arora et al., J. Phys. Condens. Matter 16, 1025 (2004).
[6] R.J. Speedy, J. Phys. Condens. Matter 8, 10907 (1996).
[7] M.G. Tucker, D.A. Keen, M.T. Dove, A.L. Goodwin, and Q. Hui, J. Phys. Condens. Matter 19, 335218 (2007).
[8] D.A. Keen, J. Appl. Crystallogr. 34, 172 (2001).
[9] M.G. Tucker, D.A. Keen, J.S.O. Evans and M.T. Dove, J. Phys. Condens. Matter 19, 335215 (2007).
Principal Publication and Authors
D.A. Keen (a), A.L. Goodwin (b), M.G. Tucker (a), M.T. Dove (b), J.S.O. Evans (c), W.A. Crichton (d), and M. Brunelli (d), Phys. Rev. Lett. 98, 225501 (2007).
(a) ISIS Facility, Rutherford Appleton Laboratory, Chilton (UK)
(b) Department of Earth Sciences, University of Cambridge (UK)
(c) Department of Chemistry, University Science Laboratories, Durham (UK)
(d) ESRF