- Home
- News
- Spotlight on Science
- Towards biological...
Towards biological imaging by coherent X-ray diffraction at the ESRF
03-03-2008
Three dimensional imaging of whole cells and other biological material will deepen our understanding of cellular biology. High-resolution imaging is needed for samples in their natural state or as close as possible to it without introducing structural artifacts by staining, sectioning, or radiation damage. The high penetrating power of X-rays could permit a cell to be imaged as a whole; a potential technique being coherent X-ray diffraction microscopy.
Share
Coherent X-ray diffraction microscopy (XDM) involves a method of image reconstruction from the coherent X-ray diffraction pattern of a sample [1]. The resolution is determined by the largest momentum transfers at which scattered radiation from the sample can be detected, rather than by a lens. For the X-ray imaging of biological samples, radiation damage usually sets the fundamental resolution limit. Here XDM is well adapted since the required radiation dose is less than in lens-based imaging where the low efficiency of optics is a limitation. An experimental demonstration by Shapiro et al. using unstained, dried yeast cells has proven the feasibility of the method in biological imaging with soft X-rays [2].
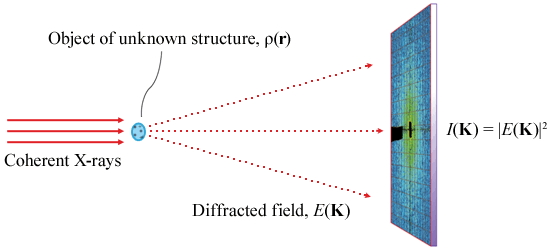
Figure 1 shows the basic XDM experimental setup. The reconstruction of the scattering length density ρ(r) is achieved by recovering the lost phases of the diffraction pattern, I(Κ), through a phase retrieval algorithm [3].
 |
|
|
Figure 1: Schematic diagram of the experimental setup for coherent diffraction imaging. |
Hard X-rays are preferable to soft X-rays in XDM since the convergence of the phasing algorithm, especially for three-dimensional reconstructions, is improved with the valid Born approximation. In addition, the sample environment is much simplified compared to the high vacuum requirements for soft X-rays. However, the scattering strength and signal-to-noise ratio from unstained biological samples illuminated with hard X-rays were unknown until recently.
Figure 2 shows the far-field diffraction patterns of unstained, frozen-hydrated Deinococcus radiodurans bacteria measured at the ID10C (Troika) beamline using 8 keV X-rays. Samples were rapidly plunge-frozen in the hydrated state to avoid structural artifacts from dehydration and to protect against radiation damage. Mounted on the diffractometer, ice contamination was avoided or reduced to a minimum by keeping the samples in a cold environment provided by a cryogenic gas stream.
No staining or sectioning of samples was employed with the few-micrometre-thick bacteria cells. Thus the contrast of the diffraction pattern arises from the biological material itself. The spatial frequencies at the edge of the diffraction pattern correspond to a resolutions in the real space of 25 nm and 29 nm for Figure 2a and Figure 2b, respectively. Phasing the diffraction patterns, and thus reconstructing the images, is currently under investigation.
 |
|
Figure 2: Coherent diffraction patterns (speckle images) of unstained Deinococcus Radiodurans bacteria, prepared in the frozen-hydrated state, imaged with 8 keV X-rays at ID10C, ESRF. |
References
[1] J. Miao, P. Charalambous, J. Kirz and D. Sayre, Nature 400 342-344 (1999).
[2] D. Shapiro et al., PNAS 102 15343-15346 (2005).
[3] J.R. Fienup, Appl. Opt. 21, 2758-2769 (1982).
Authors
E. Lima, L. Wiegart, P. Pernot, F. Zontone, M. Mattenet, E. Papillon, J. Timmins and A. Madsen, ESRF.



