- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- Energy-dispersive EXAFS to Study Chemical Reactions: the Case of the Electron-Transfer Reaction of Hydroquinone
Energy-dispersive EXAFS to Study Chemical Reactions: the Case of the Electron-Transfer Reaction of Hydroquinone
Most of the chemical processes that occur in nature take place in liquid media. For this reason, chemical reactions in solution have been widely studied for many years. Such studies have focused mainly on the mechanism of the reactions, whilst the structures of the species involved have received considerably less attention. This is mainly due to the difficulties of determining experimentally the structures of chemical species in liquid media. To date, this important question has largely been addressed by the standard techniques of UV-Vis and infrared spectroscopies. Unfortunately these techniques are seldom structurally specific, so the determination of the detailed chemical form of the majority of reactants has awaited the arrival of a fast and more structurally-focused method. Energy dispersive X-ray absorption spectroscopy (EDXAS), is ideal for this new class of experiments.
The key strength of this technique as used at beamline ID24 is its ability to collect spectra on timescales as fast as a millisecond, yielding all the characteristic information that can be obtained by conventional X-ray absorption spectroscopy, i.e. the specific local structure centred on a selected atomic species, such as inter-atomic distances, number and type of neighbouring atoms, and degree of thermal and structural disorder.
 |
| Fig. 36: Oxidation reaction of hydroquinone, showing the two different steps proposed. |
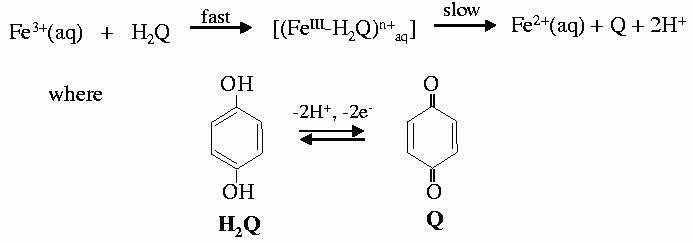
As an example we followed the redox reaction of hydroquinone with iron(III) perchlorate as the oxidising reagent to form quinone. This reaction involves both electrons and protons coming from hydroquinone (Figure 36). In concentrated solutions the formation of a blue intermediate species has been observed by uv-vis spectroscopy and is easily corroborated by direct observation [1]. The reaction was followed by XAFS spectra collected at the Fe K-edge on beamline ID24. Different pH values of 1.0, 1.3, 1.6 and 1.9, were used to monitor their influence on the rate of the reaction. For each pH value, 15 spectra were collected as the reaction proceeded and each spectrum was collected in 50 ms. The series of spectra taken for the two extreme pH values are shown in Figure 37. The reaction is faster when the concentration of protons is lower, in good agreement with the mechanism proposed and with previous observations. However, the first step of the reaction and the proposed intermediate were not detected at any of the pH values. In an attempt to observe it, fast spectral series at 10 and 1 ms per spectrum were taken. The initial state and the first spectrum of the series were found to be identical, in contradiction with the results of rapid-scan spectroscopy - the blue long-lived intermediate has a lifetime of the order of 5ms [2]. The explanation for this apparent contradiction is found in the structure of this intermediate. The substitution of a water molecule for hydroquinone does not produce a significant change in the electronic environment of Fe, and hence does not significantly perturb the XAFS spectrum.
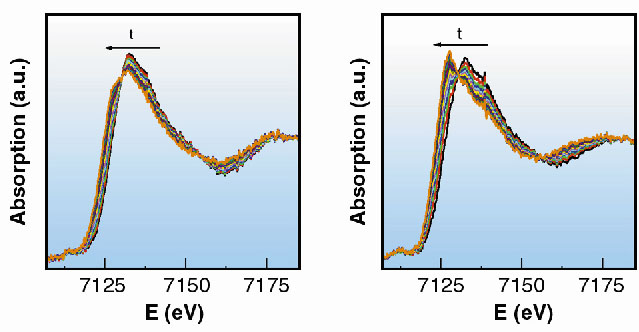 |
| Fig. 37: Series of XANES spectra taken in 50 ms each at different values of pH, corresponding to pH values of 1 and 1.9 (a and b). |
A series of 20 spectra was collected, following the reaction to completion. Each spectrum was acquired in 270 ms, with the time between two consecutive spectra set to 500 ms (Figure 38). We can conclude that the reaction is finished approximately 13 s after the mixing of the reactants.
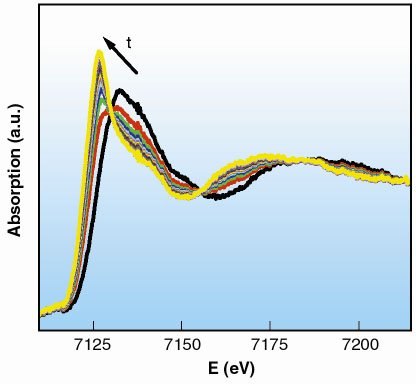 |
Fig. 38: XANES region of the spectra collected in 270 ms. |
The XANES region of the first and the last spectra of the series are identical to the initial and final state of the reaction, Fe(III) and Fe(II) respectively. These spectra correspond to an octahedral environment of water molecules around the central cation with distances Fe-O equal to 2.02 Å and 2.10 Å. The analysis of the time-resolved series shows the relative proportions of Fe(III) and Fe(II) as a function of time through a linear combination of these initial and final state spectra.
References
[1] B.S. Brunschwig, C. Creutz, D.H. Macartney, T-K. Sham and N. Sutin., Faraday Discuss. Chem. Soc., 74, 113 (1982).
[2] A. Haim, Acc. Chem. Res., 8, 264 (1975).
Authors
S. Diaz-Moreno (a), J. Evans (b), J.I. Perruchena (c), I. Diaz (d) and T. Neisius (a)
(a) ESRF
(b) Univ. Southampton (UK)
(c) Instituto de Ciencia de Materiales de Sevilla, CSIC (Spain)
(d) Instituto de Biologia Vegetal y Fotosintesis, CSIC (Spain)



