- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- X-ray imaging
- Imaging scale-free structural organisation of oxygen interstitial atoms favouring high temperature superconductivity
Imaging scale-free structural organisation of oxygen interstitial atoms favouring high temperature superconductivity
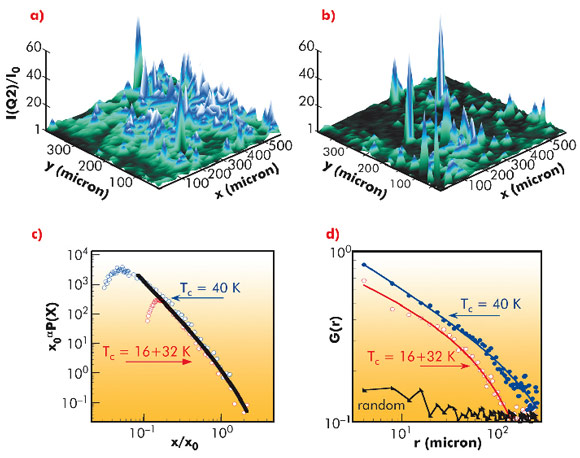
A new experiment using a focussed micro X-ray beam has found a surprising pattern lurking in a high temperature superconductor showing that high temperature superconductivity belongs to the class of collective “Quantum Phenomena in Complex Matter”. The experiment was performed at beamline ID13. We studied a layered oxide of copper belonging to the class of metallic ceramics that held the record for operating at the highest temperature when researchers discovered the superconductivity of this material. Oxide-based superconductors are very difficult to study owing to their extra (interstitial) or missing (vacancy) oxygen atoms, called dopants, which are known to roam around in the skeleton of the material, formed by other elements, and that may freeze in ordered or random patterns when the samples are cooled. The reason for this material’s elevated high-temperature conductivity was, until now, not known. For many years scientists assumed that it was because of a homogeneous distribution of dopants, which made researchers concentrate on the nanometre arrangement of these dopants to find the answer to the superconductivity. We have focussed on structure at the nanometre scale as the determinant of the unusually strong superconductivity of the oxides of copper. We used the new technique of X-ray microscopy to examine a copper oxide superconductor whose internal structure could be changed via simple heat treatments – an approach employed by ceramicists over millennia to modify oxide materials. We discovered that the best superconductivity was obtained when the microstructure was most ‘connected’, meaning that it is possible to trace a path with the same nanostructure (exhibited by oxygen atoms) over a large distance. The microstructure in this case was ‘fractal’: if we were to zoom in on the material’s structure at increasing levels of magnification, its appearance would remain the same (see Figure 132).
To see whether the fractal pattern was important, we interfered with it by heating and then quickly cooling the superconductor. Crystals with stronger fractal patterns performed better as a superconductor at higher temperatures than those with weaker fractal patterns. The high temperature conductivity was promoted by oxygen-crystal defects that form geometrical patterns that look the same on different scales, ranging from a micrometre up to fractions of a millimetre (see Figure 132). Figuring out why the fractal pattern forms in these copper-oxide crystals and how it influences the superconductivity are the next challenges.
Once the details are uncovered, researchers could control the arrangement of oxygen atoms to design better copper-oxide superconductors — perhaps even those that operate at room temperature.
Principal publication and authors
M. Fratini (a,b), N. Poccia (a), A. Ricci (a), G Campi (a,c), M. Burghammer (d), G. Aeppli (e) and A. Bianconi (a), Nature 466, 841-844 (2010).
(a) Physics Department, Sapienza University of Rome (Italy)
(b) Current address: Institute for Photonic and Nanotechnologies, CNR, Roma (Italy)
(c) Institute of Crystallography, CNR, Roma (Italy)
(d) ESRF
(e) London Centre for Nanotechnology and Department of Physics and Astronomy, University College London (UK)