- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Matter at extremes
- Bimetallic nanoalloys as highly effective hydrogenation catalysts
Bimetallic nanoalloys as highly effective hydrogenation catalysts
The sugar-derived compounds levulinic acid and γ−valerolactone are expected to play a major role in future schemes for the more sustainable production of both fuels and chemicals. Bimetallic catalysts, if properly prepared, were shown to hold much potential for the conversion of levulinic acid to γ-valerolactone, a key conversion step in biorefinery operations.
Many different types of biomass feeds and many conversion routes, involving numerous different building blocks and end products, are currently being explored for the production of bio-based chemicals. Nonetheless, a limited number of building blocks, so-called platform molecules, are emerging with the potential to play a central role in future biorefinery schemes. Levulinic acid, which is easily obtained from the carbohydrate fraction of (preferably lignocellulosic) biomass, is one such platform molecule and can be catalytically converted to a multitude of value-added products, including polymer monomers, solvents, plasticisers and fuel components. In turn, the production of many of these end products involves the compound γ-valerolactone, which is obtained by hydrogenation of levulinic acid and is in itself a valuable platform molecule. The hydrogenation of levulinic acid to g-valerolactone is typically and most conveniently done with heterogeneous, supported metal-based catalysts, in particular Ru-based ones, and molecular hydrogen.
 |
|
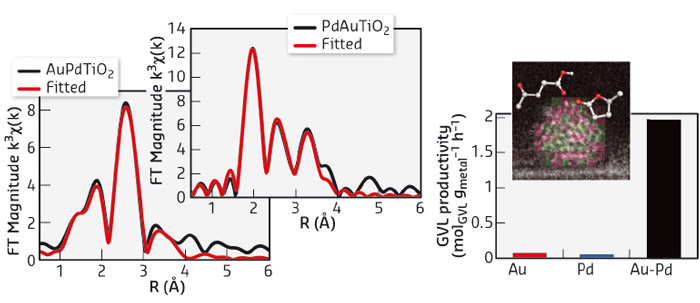
Fig. 110: Bimetallic catalysts show much improved performance in the selective hydrogenation of levulinic acid. EXAFS analysis confirmed the bimetallic nature of the nanoalloys, shown here for the Au-Pd/TiO2 catalyst, which provides a remarkable improvement in productivity compared to its monometallic counterparts. |
The use of supported bimetallic heterogeneous catalysts can be advantageous, as combinations of two metal in the appropriate proportion and with the nanostructure can lead to superior catalytic performance, in terms of activity, selectivity and/or stability of the catalyst material. Even though such nanoalloyed bimetallic catalysts might have many advantages, so far, their exploitation has been limited for biomass reactions, such as the conversion of levulinic acid to γ-valerolactone. The performance of supported bimetallic nanoparticles is governed by their composition and structural characteristics, which are in turn ultimately determined by the preparation method. It was previously shown that an ‘excess-anion’, modified impregnation method allowed for the synthesis of supported Au-Pd nanoparticles. The nanoparticles are characterised by a narrow size distribution and a quite homogeneous random nanoalloy structure [1]. We have now demonstrated that the controlled preparation of supported Au-Pd as well as Ru-Pd nanoalloy nanoparticles via this modified impregnation method provides access to catalyst materials that show markedly improved performance in the selective hydrogenation of levulinic acid to γ-valerolactone. Indeed, nanoalloying was found to positively affect the activity and selectivity as well as the stability of the bimetallic catalysts. For example, Au-Pd/TiO2 showed good activity and excellent selectivity in the hydrogenation reaction, whereas its monometallic counterparts only showed negligible activity under identical conditions. The modified impregnation method was subsequently successfully extended to give a supported Ru-Pd nanoalloy catalyst, which proved to be more selective and more stable upon reuse than its monometallic Ru/TiO2 counterpart. Extensive characterisation of the bimetallic catalysts was done by a combination of aberration-corrected scanning transmission electron microscopy (STEM), X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy after CO adsorption (FT-IR/CO) and X-ray spectroscopy (XAS). Taken together, the STEM and extended X-ray sbsorption fine structure (EXAFS) measurements, performed at beamlines BM26A and BM23, showed that the nanoparticles are indeed bimetallic and have a random nanoalloy structure (Figure 110). While the bimetallic nature of the Au-Pd catalyst was immediately evident from the Fourier Transform data at both edges, the similarity in X-ray scattering efficiency of Ru and Pd precluded distinguishing between these two metal components. For the latter sample, however, a first shell analysis (on the basis that the STEM results confirmed the bimetallic nature) did indicate intimately mixed bimetallic particles. The XPS and FT-IR/CO data provided further insight into the consequences of the nanoalloying, allowing two distinct effects to be discerned. For the Au-Pd based catalyst, the remarkable increase in activity was attributed to electronic modification of Pd upon alloying with Au. The improved stability and selectivity of the Ru-Pd catalyst were in turn attributed to the dilution and isolation of Ru by Pd in the random nanoalloy. These results highlight the potential of using bimetallic catalysts for the production of bio-based fuels and chemicals, provided that use is made of carefully designed synthesis strategies.
Principal publication and authors
High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to g-valerolactone, W. Luo (a), M. Sankar (a), A.M. Beale (b,c), Q. He (d), C.J. Kiely (d), P.C.A. Bruijnincx (a) and B.M. Weckhuysen (a), Nat. Commun. 6, 6540 (2015); doi: 10.1038/ncomms7540.
(a) Inorganic Chemistry and Catalysis, Utrecht University (The Netherlands)
(b) UK Catalysis Hub, Research Complex at Harwell, Rutherford Appleton Laboratory, Oxford (UK)
(c) Department of Chemistry, University College London (UK)
(d) Department of Materials Science and Engineering, Lehigh University, Bethlehem (USA)
References
[1] M. Sankar et al., ACS Nano 6, 6600-6613 (2012).



