- Home
- News
- General News
- A grand year for...
A grand year for European structural biology
27-10-2011
For the first time ever, more than 1000 protein structures have been deposited in a single year in the Protein Data Bank (PDB) from data collection at the ESRF.
Share
The ESRF’s structural biology beamlines resemble a production line for protein crystal analysis and data collection. Today, 25/10/2011, the number of structures deposited in the Protein Data Bank (PDB) [1] for the year 2010 reached 1005 following data collection at the ESRF. An impressive result on its own, but this is just the tip of the iceberg; below the surface, there were many more data sets collected (21,540) from a total of 123,309 crystals studied in 2010. At a European level, the ESRF accounts for 47% of the total synchrotron output to date. These deposition statistics were provided by the BioSync website [2].
The majority of the structures were solved from data collected on the six protein crystallography beamlines run by the ESRF. Significant contributions also came from collaborating research group (CRG) beamlines such as BM14, BM16 and BM30A.
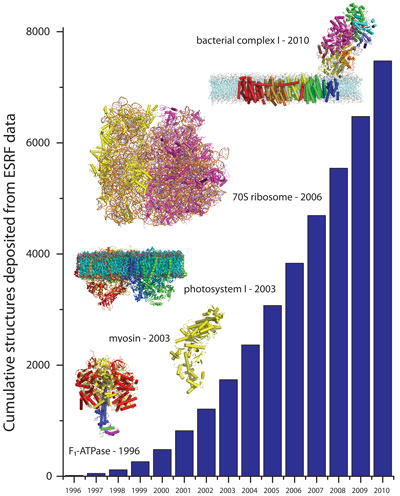 |
|
Structures deposited in the PDB from data collected at the ESRF; some significant structures solved over the years are also shown. |
While the total number of structures solved has increased exponentially, so too has the complexity of the proteins being studied (see Figure). Sean McSweeney, head of the ESRF Structural Biology group explained, “Fifteen years ago the definition of a challenging project was different, today the structures solved are often more complex multisubunit or membrane bound proteins”. Each structure solved has contributed to our knowledge of the functioning at the cellular level of the human body or of other animals, plants, bacteria and viruses, facilitating the development of new drugs and medical treatments.
During the past 15 years, initiatives such as the EU funded projects SPINE, SPINE 2 [3] and BIOXHIT [4] have facilitated production and purification of proteins. Continuous innovation at the ESRF beamlines (often carried out in collaboration with staff of the EMBL Grenoble Outstation) has led to higher throughput with better and more reliable data collection. Notable developments have been the generalisation of the use of a cryostream to protect crystals from radiation damage; robotic sample changers for high throughput; automated routines for beamline and crystal alignment; computer assisted identification of the best crystals or regions within a crystal for data collection (diffraction cartography) and the addition of microfocus beamlines for data collection from ever smaller crystals. However, we are not resting on our laurels. Currently under construction within the ESRF Upgrade Programme is the next generation of beamlines for integrated Structural Biology. The project, called MASSIF, will see a new beamline complex built at ID30. The new beamlines will be optimised for even higher throughput, with advanced robotics and automation. These beamlines are currently under commissioning, and their first users are expected in 2013.
“Second generation automation will make the new beamlines even more efficient and allow even the most challenging projects to become feasible”, promises Matthew Bowler, scientist responsible for the MASSIF automated sample selection beamlines.
References
[1] PDB: http://www.pdb.org/
[2] Statistics provided by BioSync: http://biosync.sbkb.org/
[3] SPINE (Structural Proteomics in Europe) and SPINE 2 - Complexes are now superseded by INSTRUCT: http://www.structuralbiology.eu/
[4] BIOXHIT (Biocrystallography (X) on a Highly Integrated Technology Platform for European Structural Genomics): http://www.bioxhit.org/
Top image: Each year the solved protein structures are becoming more complex.



