- Home
- News
- Spotlight on Science
- Nanoparticle behaviour...
Nanoparticle behaviour in changing environments: dynamic structural reorganisation in supported Pd nanoparticles during redox cycling
18-10-2007
The size and shape of supported Pd nanoparticles has been found to change rapidly with the environment they experience. Somewhat unexpectedly, even in the presence of large amounts of oxygen, significant oxidation of the Pd does not appear to be the principal reactive event. Instead, our results indicate that the first consequence of Pd interacting with oxygen is a dramatic decrease in average Pd particle size.
Share
Supported nanoparticulate noble metals, such as palladium (Pd), are widely used as heterogeneous catalysts - perhaps the most well-known application being in the cleaning up of exhaust gas emissions. As oxygen is omnipresent within this and other important catalytic scenarios where Pd is used, the behaviour of oxidic and metallic nanosize Pd species is of great interest. The environment these catalysts work within often changes very rapidly (for instance in a car exhaust), hence understanding how these phases might form and interconvert in response to this variability is potentially very important indeed. Using dispersive EXAFS, a direct, time resolving (in this case 3-4 Hz) probe of local structure, we can investigate these processes in situ.
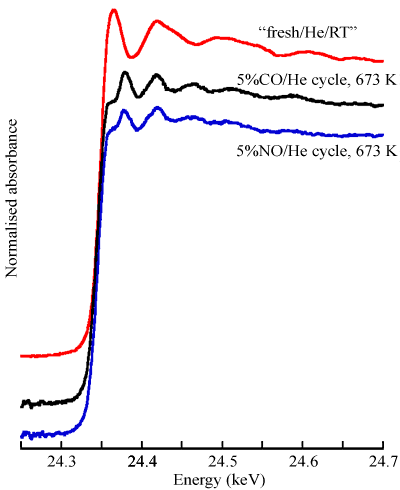
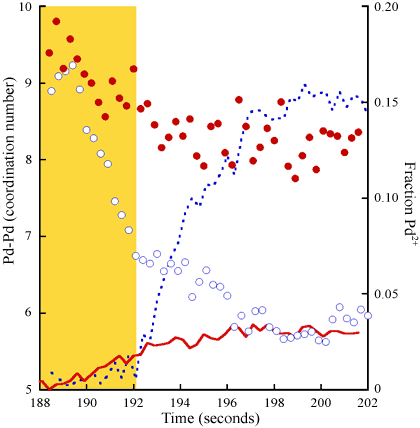
Experiments were carried out at beamline ID24. Dispersive EXAFS data were recorded for a 1 wt% Pd catalyst during a cycling experiment between flows of 5%CO and 5%NO/He. Figure 1 presents 300 millisecond spectra at different stages in a single redox cycle. Analysis of such spectra leads to Figure 2, where the changes in Pd-Pd co-ordination number and the levels of oxidised Pd (fraction of Pd2+) formed during a single redox cycle are plotted for two oxidising environments.
P
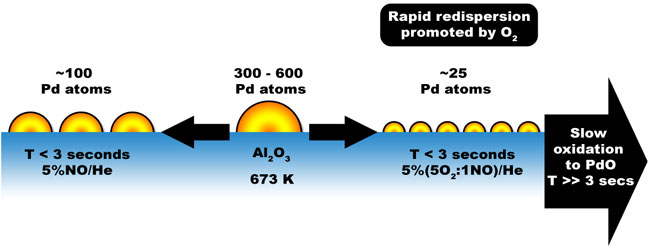
Using this approach we can clearly distinguish events related to size and shape change from those due to Pd oxidation. The co-ordination number (CN) is, in this case, indicative of the number of Pd atoms present in an average nanoparticle and its variation shows that supported Pd particles of up to 3 nm in diameter rapidly (within 3 seconds) and reversibly alter their size/shape distribution in response to their environment. What is further remarkable is that oxygen actually promotes this very significant structural change before any significant oxidation of the Pd (to Pd2+) is observed. Figure 3 summarises the results obtained.
 |
|
Figure 3: Size/shape changes of the Pd nanoparticles under the influence of the gasous composition of their atmospheres. |
Such rapid redispersion of metallic nanoparticles that proceeds via an apparently non oxidative route, and indeed prior to oxidation itself (shaded region of Figure 2), has not previously been demonstrated.
How these fundamental nanoscale rearrangements contribute to the overall chemistry mediated by such catalysts, and whether these new processes can in themselves be manipulated for practical benefit, are themes of continuing study.
Principal Publications and Authors
M.A. Newton (a), C. Belver-Coldeira (b), A. Martínez-Arias (b), M. Fernández-García(b), Nature Materials, 6, 528-532 (2007); and Angewandte Chemie (2007), in press.
(a) ESRF
(b) Instituto de Catalisis y petroleoquimica, Madrid (Spain)